ACE inhibitory peptide and preparation method and application
A technology for inhibiting peptides and inhibiting activity, which is applied in the field of ACE inhibitory peptides and preparations, and can solve problems such as taste disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of natural ACE inhibitory peptide
[0026] 1. Extraction of sea cucumber protein: Take 200g sea cucumber and dissolve it in 2000mL pre-cooled 0.1mol / L NaOH solution. After the tissue is homogenized, centrifuge (12000g, 4°C, 20min), and the obtained precipitate continues to be dissolved in NaOH buffer. After stirring overnight, centrifuge (12000g, 4°C, 20min) to collect the precipitate. After repeated 4 times, the precipitate collected after the last centrifugation was repeatedly rinsed with distilled water until the pH of the eluate was neutral. After washing with water, the protein was dissolved in 10 times the volume of 0.5 mol / L acetic acid, stirred overnight and then centrifuged (12000g, 4°C, 20min), the obtained supernatant was the sea cucumber protein solution.
[0027] 2. Enzymatic hydrolysis of sea cucumber protein: Preheat the sea cucumber protein solution in a 37°C water bath for 5 minutes, add 0.5% by weight of pepsin and place it a...
Embodiment 2
[0030] Example 2: Preparation of artificially synthesized ACE inhibitory peptides
[0031] 1. Weigh 0.3mmol of Fmoc-Pro-Wang Resin (for P1900520) or Fmoc-Arg(Pbf)-Wang Resin (for P1900521) with a substitution degree of 0.35mmol / g, place it in a medium-sized reaction column, soak in DMF for 120min ;
[0032] 2. Drain the DMF, add 20% Pip / DMF which is 3 times the volume of the resin, and blow nitrogen for 30 minutes to remove Fmoc, wash with DMF 5 times, and the ninhydrin detection shows dark blue;
[0033] 3. Put in the raw materials in proportion (AA:HBTU:NMM=3:2.85:6), add an appropriate amount of DMF, and blow nitrogen to react until the ninhydrin detection is transparent;
[0034] 4. Repeat steps 2-3 to complete the sequence synthesis;
[0035] 5. Drain the resin in the reactor, transfer it to a cutting tube, add 40ml of F solution, and control the temperature on a shaking table for 1.5h;
[0036] 6. Suction filtration, collect the cutting filtrate into a centrifuge tube...
Embodiment 3
[0039] Example 3: Detection of natural ACE inhibitory activity
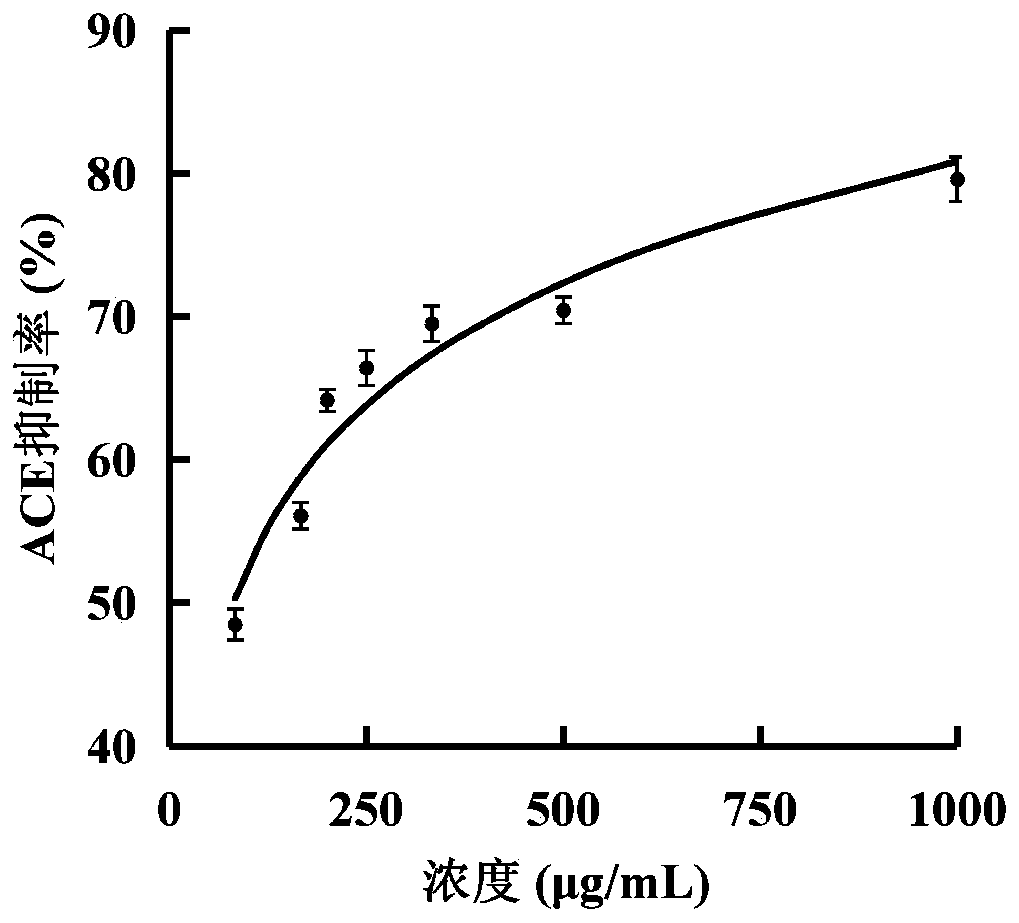
[0040]The permeate obtained after the enzymolysis solution is separated by ultrafiltration with a 3kDa ultrafiltration membrane is lyophilized and concentrated to obtain a lyophilized powder. After diluting into different concentrations with water, the ACE inhibitory activity was detected, and the results were as follows: figure 1 shown. It can be seen from the figure that the ACE inhibitory activity IC of the permeate 50 It was 81.37 μg / mL. The naturally-prepared ACE inhibitory peptide is obtained by enzymatic hydrolysis with gastrointestinal protease, which can prevent the active peptide from being degraded and inactivated by the digestive juice of the gastrointestinal tract after being ingested by the human body.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com