Acidic mammal chitinase coding gene and application
A technology of chitinase and coding gene, applied in plant gene improvement, application, genetic engineering, etc., can solve the problems of low activity, large amount of acidic mammalian chitinase expression, low expression level, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, acidic mammalian chitinase gene sequence codon optimization
[0028] The gene sequence of mammalian chitinase derived from mouse stomach tissue was optimized to a certain extent by codon: a, reducing the occurrence probability of continuous A / T / G / C bases, and avoiding the generation of stem-loop structure; b, Throughout the gene sequence, some rare codons were added, especially at the initiation of translation, to reduce the rate of ribosome elongation. The optimized acidic mammalian chitinase gene sequence is shown in SEQ ID NO.2, and the optimization rate of the whole sequence is 23.4% compared with the original sequence.
Embodiment 2
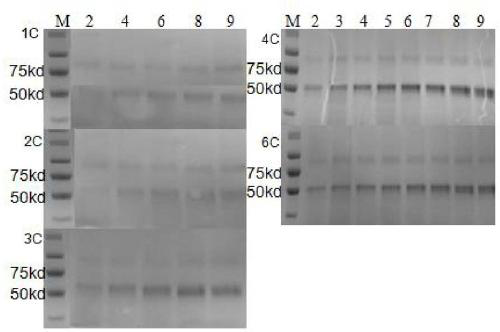
[0029] Embodiment 2, construction of expression vector and protein expression
[0030] 1. Artificial synthesis of gene sequence
[0031] The nucleotide sequence shown in SEQ ID NO.2 was entrusted to Wuhan Jinkairui Bioengineering Co., Ltd. to artificially synthesize the gene according to the conventional technology in the field, and the gene was inserted into the plasmid vector pUC57, and stored for future use.
[0032] 2. Gene sequence amplification
[0033] Design primer pair (Dchit-F, Dchit-R) according to the nucleotide sequence shown in SEQ ID NO.2
[0034] The underlined part of the forward primer is the Cpo I restriction site, the underlined part of the reverse primer is the Not I restriction site, and the sequence design of this site conforms to the T 4 Cohesive ends produced by DNA polymerase trimming.
[0035] PCR reaction system:
[0036]
[0037]
[0038] PCR reaction conditions: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 5 s, annealing...
Embodiment 3
[0050] Example 3, construction of cofactor genes and related vectors
[0051] 1. Cofactor gene amplification
[0052] Through the website https: / / www.ncbi.nlm.nih.gov / , find the Mxr1, Hac1 and Pdi1 gene sequences derived from Pichia pastoris GS115 and design primers for gene amplification (primer list), these genes were respectively constructed in the pGAPZB vector between the EcoR I and Age I restriction sites.
[0053] PCR reaction system:
[0054]
[0055] PCR reaction conditions: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 5 s, annealing at 55°C for 5 s, extension at 72°C for 10 s, 30 cycles of amplification, and final extension at 72°C for 10 min.
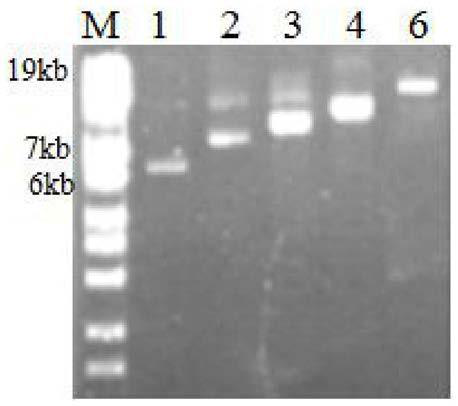
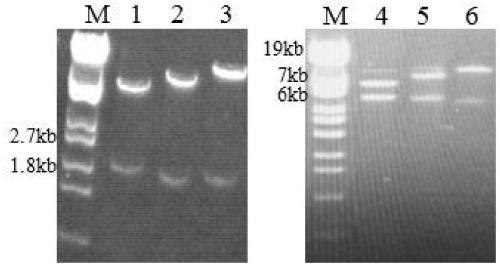
[0056] The PCR product was detected by 0.7% agarose gel electrophoresis, and purified with a DNA purification kit (produced by GeneMark).
[0057] 2. Construction of recombinant expression vector
[0058] 1) The plasmid pGAPZB was double-digested with EcoR I and Age I, and the digested product was recov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com