Antibacterial preservative film, and preparation method and applications thereof
A fresh-keeping film and a technology for its use are applied in the field of fresh-keeping films containing lauroyl arginine ethyl ester salt and derivatives thereof, and achieve the effects of simple preparation, good stability and easy biological catabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation method of synthetic ion-pair compound of lauroyl arginine ethyl ester hydrochloride and nicotinic acid
[0065] Dissolve 2.0 g of sodium nicotinic acid (purchased from Tixiai (Shanghai) Chemical Industry Development Co., Ltd.) in 50 mL of water to prepare sodium nicotinic acid salt solution (A); 6.8 g of lauroyl arginine ethyl ester hydrochloride Dissolve in 40mL of water and heat to 90°C until all lauroyl arginine ethyl ester hydrochloride is dissolved to prepare an aqueous solution of lauroyl arginine ethyl ester hydrochloride (B); The saline solution (A) is slowly added to the lauroyl arginine ethyl ester hydrochloride solution (B), stirring constantly, reacting for 2 hours, cooling to room temperature, filtering, fully washing the precipitate with purified water, and drying the precipitate in vacuum at 60°C. 7.6 g of niacin ion pair compound is obtained.
Embodiment 2
[0066] Example 2: Analysis of the molecular formula and molecular weight of the compound of the nicotinic acid ion of ethyl lauroyl arginine
[0067] By mass spectrometry, 1 H-NMR, 13 The molecular formula of the compound obtained by C-NMR spectrum analysis is:
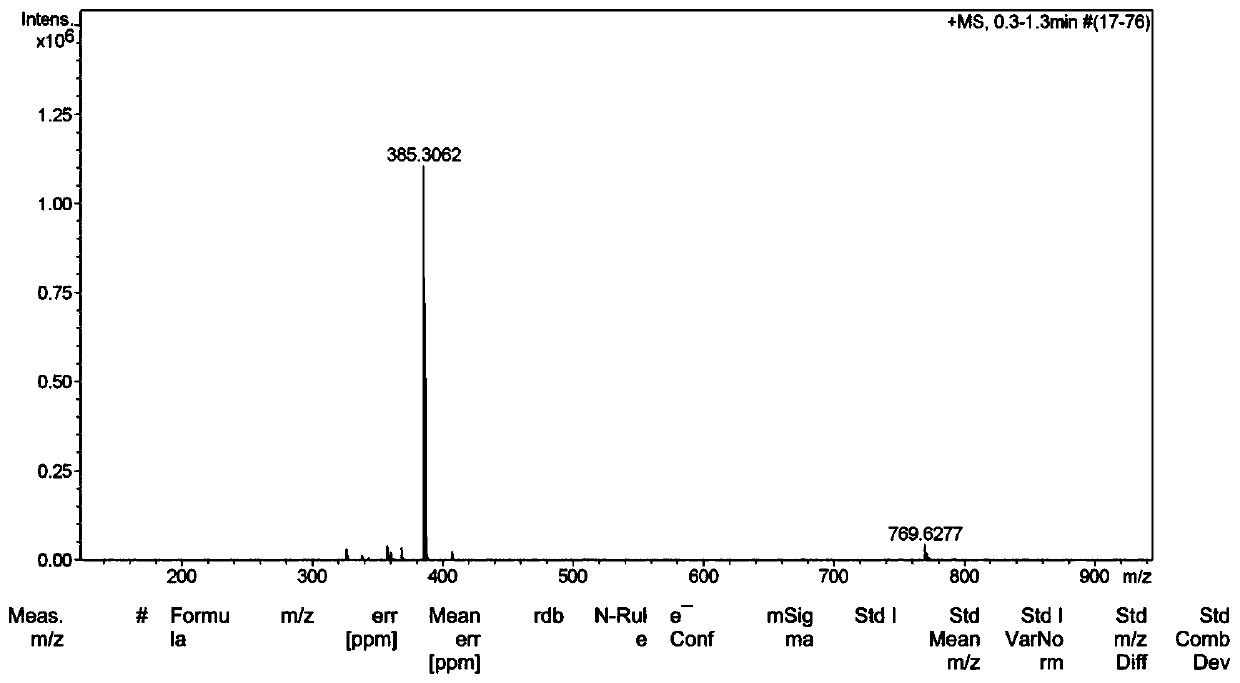
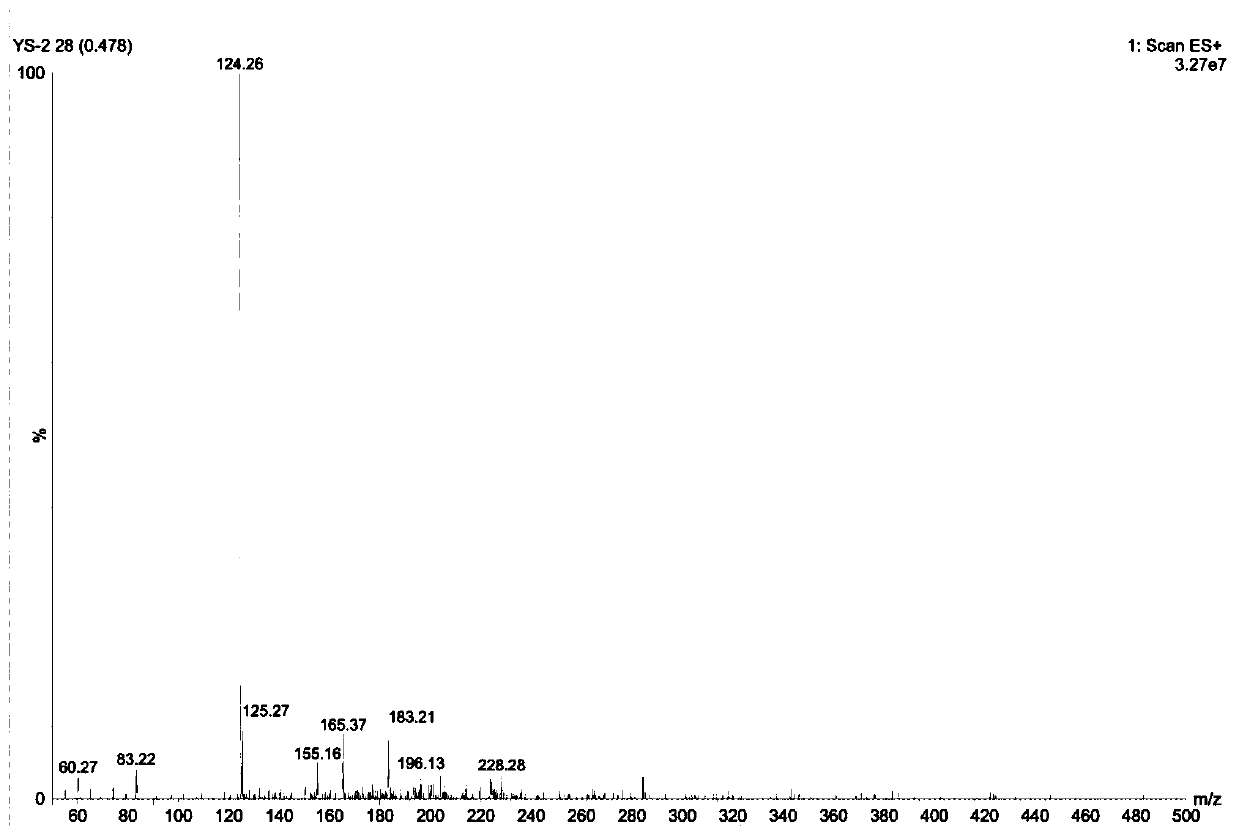
[0068] 1. Mass spectrometry (ESI) analysis
[0069] Cation B + The m / z of the molecular ion peak = 385.3, see figure 1 ;
[0070] Anion A - The m / z of the molecular ion peak = 122.1, see figure 2 .
[0071] The theoretical calculated value of the cation of the niacin ion pair compound is 507.4, and the measured value is consistent with the theoretical value.
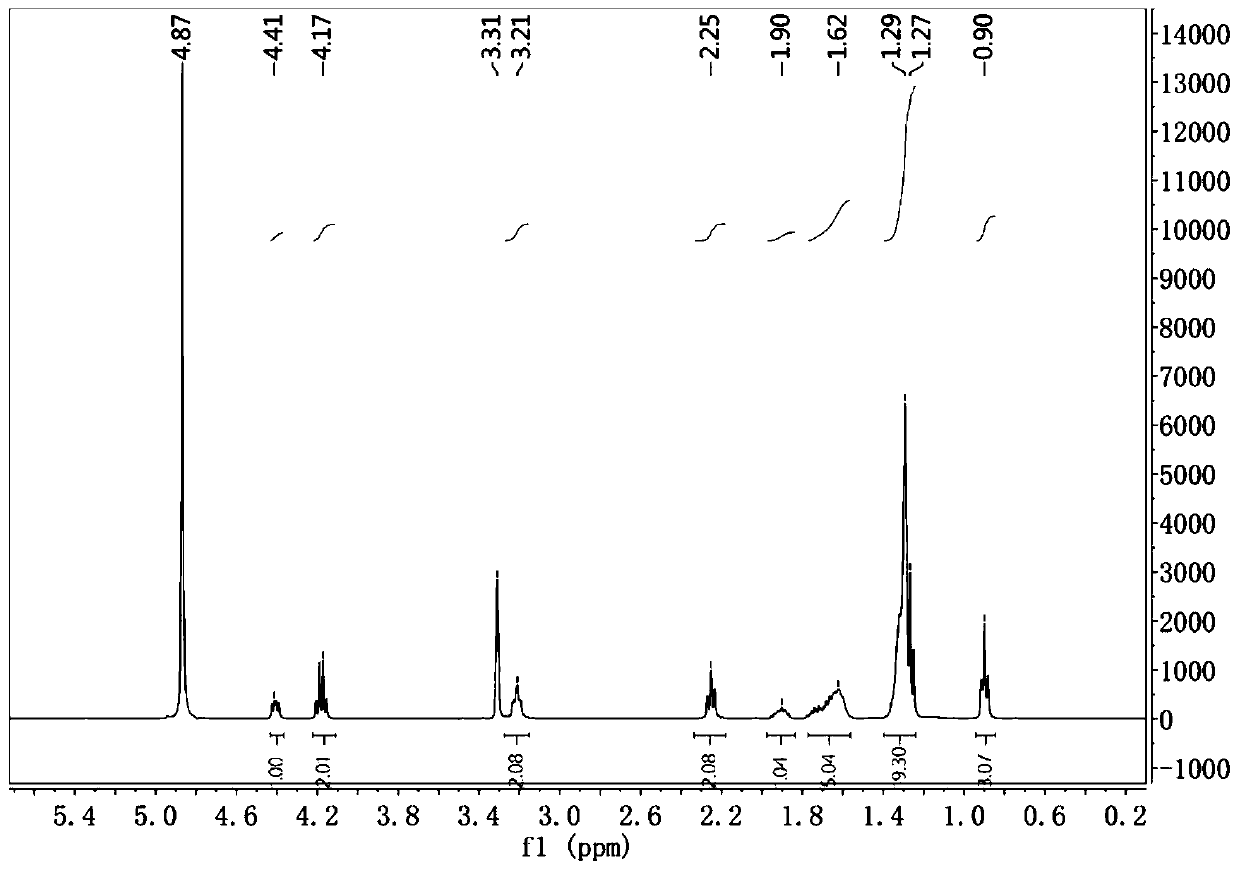
[0072] 2.NMR analysis
[0073] Lauroyl arginine ethyl ester hydrochloride (see image 3 ), niacin 1 H-NMR (see Figure 4 ) And LAE niacin ion pair compound 1 H-NMR (see Figure 5 )Compared. Since the LAE ion-pair compound is in the process of salt formation, the peak shape and chemical shift of the ion-pair compound of lauroyl arginine ethyl ester change little, but all h...
Embodiment 3
[0074] Example 3: Preparation method of synthetic ion-pair compound of lauroyl arginine ethyl ester hydrochloride and tartaric acid
[0075] Dissolve 2.0 g of tartaric acid (purchased from Tixiai (Shanghai) Chemical Industry Development Co., Ltd.) in 50 mL of methanol, add equivalent NaOH, stir at room temperature until a white solid precipitates, filter with suction and wash with 30 mL of methanol three times to obtain sodium tartrate . Sodium tartrate is dissolved in 50mL of water to prepare a sodium tartrate solution (A); 5.6 g of lauroyl arginine ethyl ester hydrochloride is dissolved in 40 mL of water and heated to 90°C until the lauroyl arginine ethyl ester salt The acid salt is completely dissolved to prepare an aqueous solution of lauroyl arginine ethyl ester hydrochloride (B); the sodium tartrate solution (A) is slowly added to the aqueous solution of lauroyl arginine ethyl ester hydrochloride ( In B), continue to stir, react for 2 hours, cool to room temperature, filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com