Engineered artificial antigen presenting cells for tumor infiltrating lymphocyte expansion
一种人工抗原、细胞的技术,应用在受体/细胞表面抗原/细胞表面决定因子、抗肿瘤药、动物细胞等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0607] Example 1 - Variability in Expansion of Tumor Infiltrating Lymphocytes Using PBMC Feeder Cells
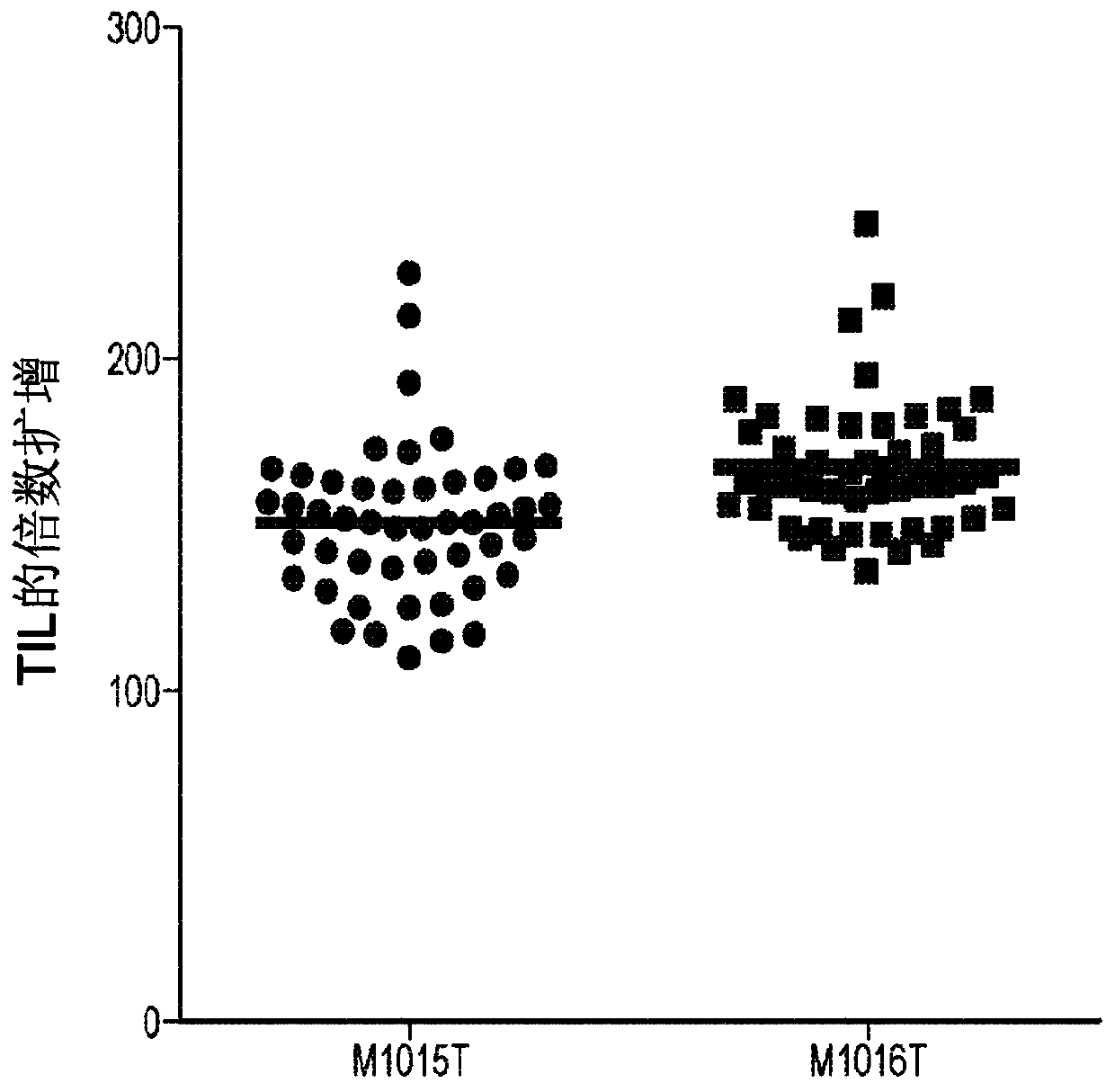
[0608] By comparing the results of multiple TIL expansions on the same TIL line obtained from a patient, the variability in TIL expansion obtained by using PBMC feeder cells can be demonstrated. figure 1 Typical results for rapid expansion of TILs using irradiated allogeneic PBMC feeder cells (PBMC feeder cells) are shown. Will be marked as M1015T and M1016T (1.3×10 5 cells) of two TIL lines with 46 different irradiated feeder cell batches (1.3 × 10 7 ), IL-2 (3000IU / mL), recombinant human IL-2 (eg, aldesleukin or equivalent) (CellGenix, Inc., Portsmouth, NH, USA) and OKT-3 (30ng / mL, MACS GMP CD3 pure, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were co-cultured in T25 flasks for 7 days. Fold expansion values of TILs were calculated on day 7. The graph shows the fold expansion values for two TIL lines in separate stimulation experiments. For each TIL line, 4...
Embodiment 2
[0609] Example 2 - Selection of bone marrow cells for aAPC development
[0610] Phenotypic characterization of various myeloid lineage cell lines to identify potential candidates for further modification of aAPCs for TIL expansion. The results are summarized in Table 5. The MOLM-14 cell line exhibited endogenous expression of CD64 and was selected for further development. The EM-3 cell line was chosen based on the observed endogenous expression of ICOS-L (which was not observed in the EM-2 cell line although taken from the same patient).
[0611] Table 5. Summary of co-stimulatory molecules endogenously expressed on candidate cell lines of aAPC. CML refers to chronic myeloid leukemia and AML refers to acute myeloid leukemia. "Population" refers to the population of cells observed to express a marker (1 / 2 population = 50%).
[0612]
Embodiment 3
[0613] Embodiment 3-preparation of MOLM-14 artificial antigen-presenting cells (aMOLM14 aAPC)
[0614] MOLM-14 cells were obtained from Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. To develop MOLM-14-based aAPCs, MOLM-14 cells were engineered with costimulatory molecules CD86 and 4-1BBL (CD137L). The human CD86 (hCD86) and human 4-1BBL (h4-1BBL) genes were cloned into commercially available PLV430G and transduced using the lentiviral transduction method with the PDONR221 vector (Invitrogen / ThermoFisher Scientific, Carlsbad, CA). , USA) co-transfection. The hCD86 and hCD137L genes were cloned into PLV430G and PDONR221 vectors using the Gateway cloning method (as described by Katzen, Expert Opin. Drug Disc. 2007, 4, 571-589). The 293T cell line (human embryonic kidney cells transformed with the large T antigen) was used for lentivirus production, transduced into MOLM-14 cells. Transfected cells were sorted (S3e Cell Sorter, Bio-Rad, H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com