Preparation method of baloxavir marboxil intermediate compound
A technology of compounds and intermediates, applied in the direction of organic chemistry, can solve the problems of polluting synthesis methods, dangers, etc., and achieve the effects of optimizing purification steps, reducing production costs and unit prices, good commercial value and industrial development potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
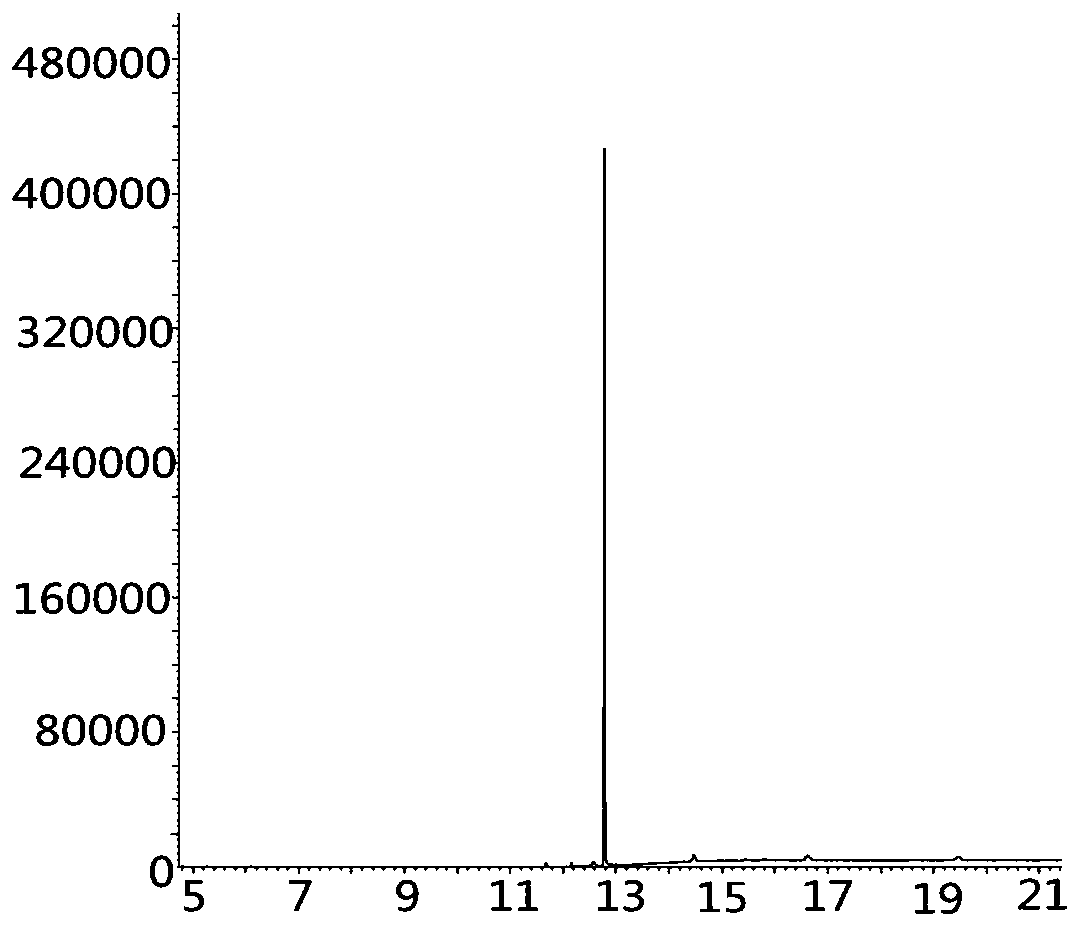
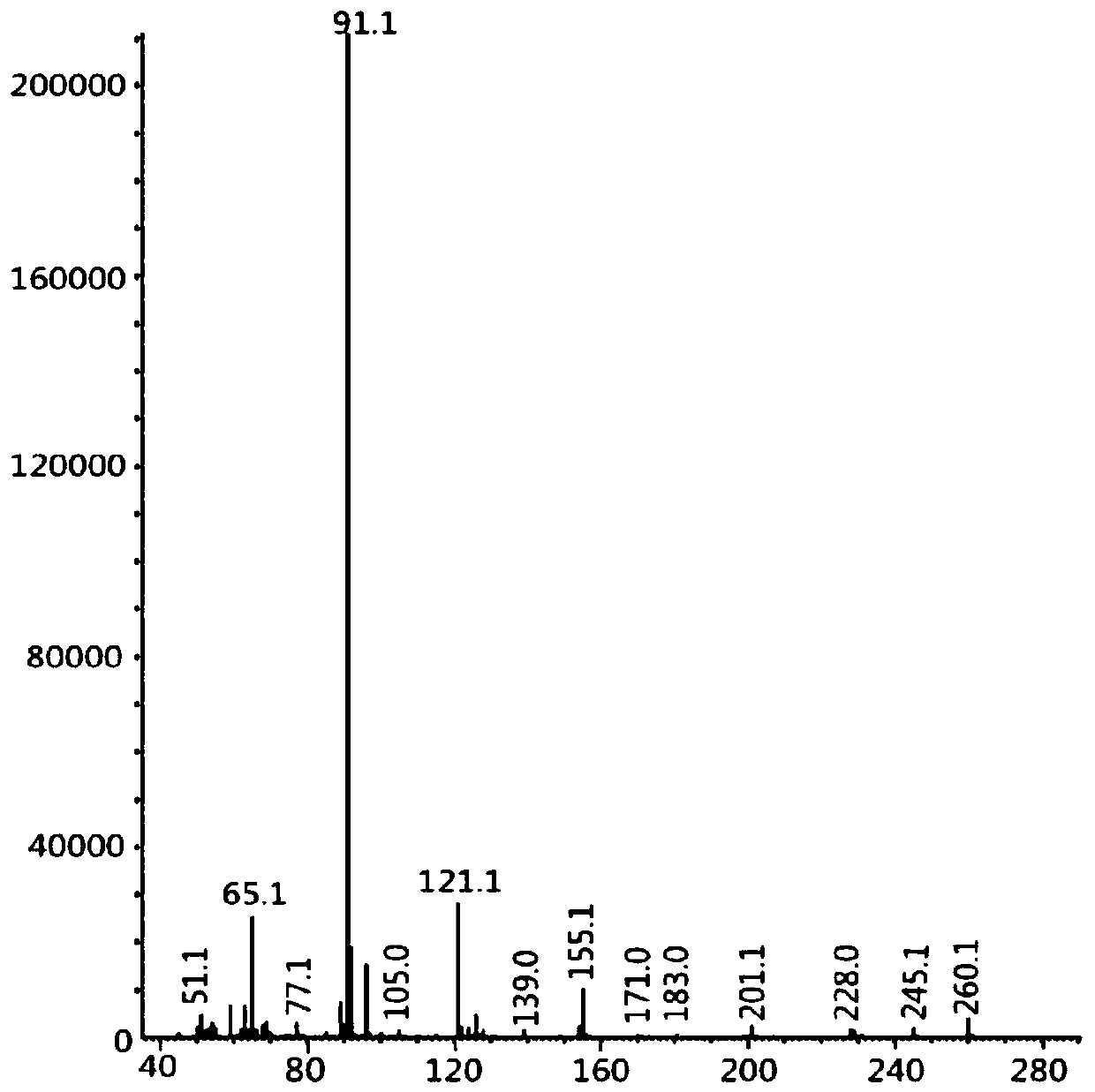
Image
Examples
Embodiment 1
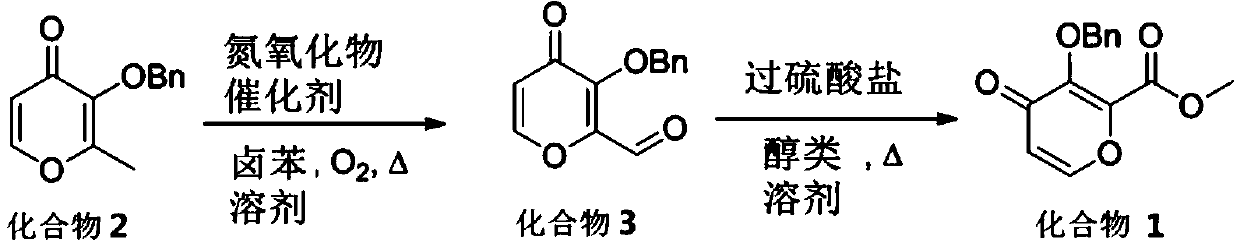
[0034] A preparation method of baloxavir intermediate compound, the baloxavir intermediate compound is 3-benzyloxy-4-oxo-4-hydropyran-2-carboxylic acid methyl ester, compound 1 Express, described preparation method is as follows:
[0035]
[0036](1), add 21.6 grams (100 millimoles) compound 2 in a 500 milliliter reaction bottle with magnetic stirring bar (can be prepared in one step by cheap and easy-to-get commercial reagent luteol, see document Organic Process Research&Development, 2012, 16 (11), 1783-1786) and 250 milliliters of bromobenzene solvents, then add 0.95 grams (10 mmol) of pyridine-N-oxides, a bottle mouth is connected with a reflux condenser, and the other bottle mouth is passed into oxygen, and then Under stirring, it was raised to 160°C to reflux the solvent, and the reaction system was kept under reflux and stirred for 20 hours. After the reaction of compound 2 was completed, it was lowered to room temperature, and then the bromobenzene solvent was separa...
Embodiment 2
[0046] A kind of preparation method of baloxavir intermediate compound, described compound is 3-benzyloxy-4-oxo-4-hydropyran-2-carboxylic acid methyl ester (compound 1), described preparation method is as follows :
[0047]
[0048] (1), add 21.6 grams (100 millimoles) compound 2 and 50 milliliters of chlorobenzene solvents in a 500 milliliters reaction bottles with magnetic stirring bar, then add 0.94 grams (6 millimoles) tetramethylpiperidine nitrogen oxidation (TEMPO), one bottle mouth is connected with a reflux condenser, and the other bottle mouth is fed with oxygen, and then raised to 140°C under stirring to make the solvent reflux reaction, keep the reaction system under reflux state and stir for 48 hours, follow the reaction of compound 2 After complete, be down to room temperature, then decompression distillation separates bromobenzene solvent and obtains residual crude product, described solvent is through drying, can reuse after redistilling, and residual crude p...
Embodiment 3
[0051] A kind of preparation method of baloxavir intermediate compound, described compound is 3-benzyloxy-4-oxo-4-hydropyran-2-carboxylic acid methyl ester (compound 1), described preparation method is as follows :
[0052]
[0053] (1), add 21.6 grams (100 millimoles) compound 2 and 25 milliliters of fluorobenzene solvents in a 500 milliliters reaction bottles with magnetic stirring bar, then add 0.35 grams (3.4 millimoles) N-methylmorpholine nitrogen For oxides, one bottle mouth is connected to a reflux condenser, and the other bottle mouth is fed with oxygen, and then raised to 100 ° C under stirring to allow the solvent to reflux for reaction, and the reaction system is kept under reflux and stirred for 36 hours. After the completion of the reaction of compound 2 , down to room temperature, and then the bromobenzene solvent is separated by distillation under reduced pressure and obtains the residual crude product. The solvent is dried and can be reused after re-evaporat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com