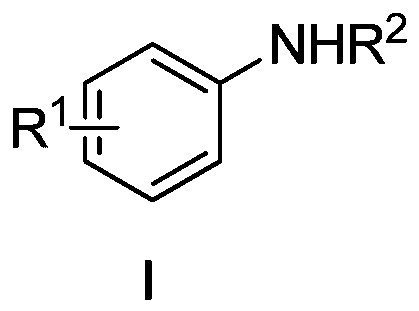
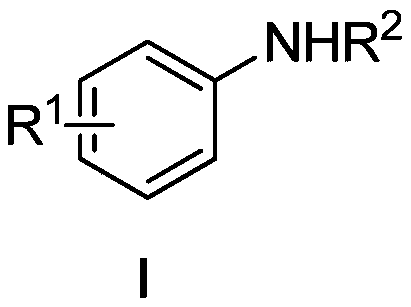
Amines based on electron-rich fluoroaromatic hydrocarbons, and preparation method of amines
A technology for fluorinated aromatic hydrocarbons and aminates, which is applied in the field of chemistry and can solve problems such as residual trace metals and harsh reaction conditions for guiding groups.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
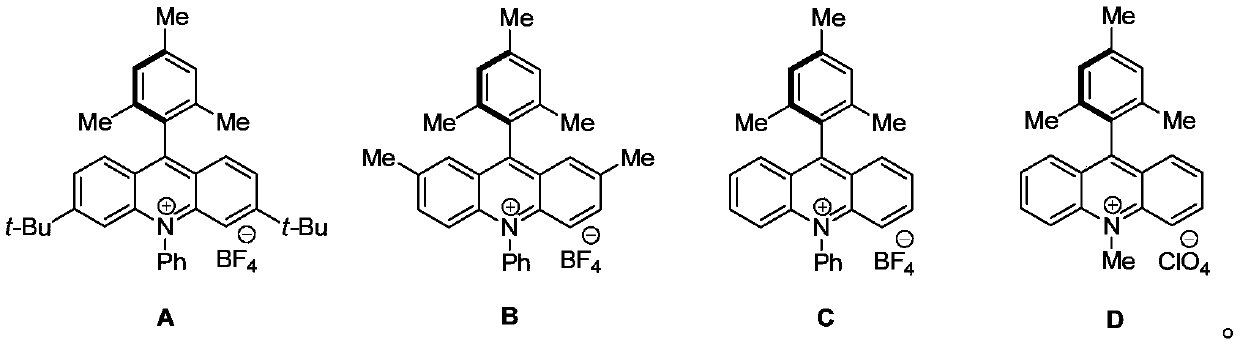
[0029] Under argon protection, add p-fluoroanisole a1 (0.0126g, 0.10mmol), acridine photosensitizer (A) (0.0029g, 0.005mmol) and L-valine ethyl ester b1 (0.0218g, 0.15mmol) ), 1,2-dichloroethane (DCE) 2.0mL. Placed under 6W blue light at room temperature to react for 48h. After the reaction, the solvent was distilled off under reduced pressure and separated by column chromatography, eluent: (V) petroleum ether / (V) ethyl acetate=20 / 1. A yellow liquid (1) (0.0208 g, 93%) was obtained.
[0030] 1 H NMR (400MHz, CDCl 3 ): δ6.77(d, J=7.6Hz, 2H), 6.62(d, J=7.6Hz, 2H), 4.17(q, J=6.4Hz, 2H), 3.87(s, 1H), 3.74(s ,3H),2.11-2.06(m,1H),1.25(t,J=6.8Hz,3H),1.05(d,J=6.8Hz,3H),1.03(d,J=6.4Hz,3H); 13 C NMR (100MHz, CDCl 3 ): δ174.0, 152.6, 141.5, 115.2, 114.8, 63.7, 60.7, 55.7, 31.5, 19.1, 18.7, 14.3; HRMS (ESI) m / z calcd for C 14 h 22 NO 3 (M+H) + 252.1594, found 252.1604.
Embodiment 2
[0032]
[0033] Under argon protection, p-fluoroanisole a1 (0.0126g, 0.10mmol), acridine photosensitizer (A) (0.0029g, 0.005mmol) and D-dimethyl aspartate b2 (0.0242g, 0.15mmol), 1,2-dichloroethane (DCE) 2.0 mL. The reaction was irradiated under 6W blue light for 48 h at room temperature. After the reaction was finished, the solvent was distilled off under reduced pressure, separated by column chromatography, eluent: (V) sherwood oil / (V) ethyl acetate=6 / 1. A yellow liquid (2) (0.0206 g, 87%) was obtained.
[0034] 1 H NMR (400MHz, CDCl 3 ): δ6.79(d, J=8.8Hz, 2H), 6.67(d, J=8.8Hz, 2H), 4.38(t, J=6.6Hz, 1H), 4.16(s, 1H), 3.74(s ,6H),3.70(s,3H),2.86(d,J=5.6Hz,2H); 13 C NMR (100MHz, CDCl 3 ): δ173.1, 171.0, 153.1, 140.2, 115.7, 114.8, 55.6, 54.8, 52.5, 52.0, 37.3; HRMS (ESI) m / z calcd for C 13 h 18 NO 5 (M+H) + 268.1180, found 268.1190.
Embodiment 3
[0036]
[0037] Under the protection of argon, add p-fluoroanisole a1 (0.0126g, 0.10mmol), acridine photosensitizer (A) (0.0029g, 0.005mmol) and L-phenylalanine methyl ester b3 (0.0269g, 0.15 mmol), 1,2-dichloroethane (DCE) 2.0 mL. The reaction was irradiated under 6W blue light for 48 h at room temperature. After the reaction, the solvent was distilled off under reduced pressure and separated by column chromatography, eluent: (V) petroleum ether / (V) ethyl acetate=10 / 1. A yellow liquid (3) (0.0237 g, 93%) was obtained.
[0038] 1 H NMR (400MHz, CDCl 3 ):δ7.23-7.20(m,2H),7.18-7.15(m,1H),7.10(d,J=6.8Hz,2H),6.69(d,J=8.0Hz,2H),6.50(d, J=7.6Hz, 2H), 4.22(t, J=6.0Hz, 1H), 3.65(s, 3H), 3.57(s, 3H), 3.04(d, J=6.6Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ173.9, 152.7, 140.4, 136.4, 129.2, 128.5, 126.9, 115.2, 114.8, 58.9, 55.6, 52.0, 38.8; HRMS (ESI) m / z calcd for C 17 h 20 NO 3 (M+H) + 286.1438, found 286.1445.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com