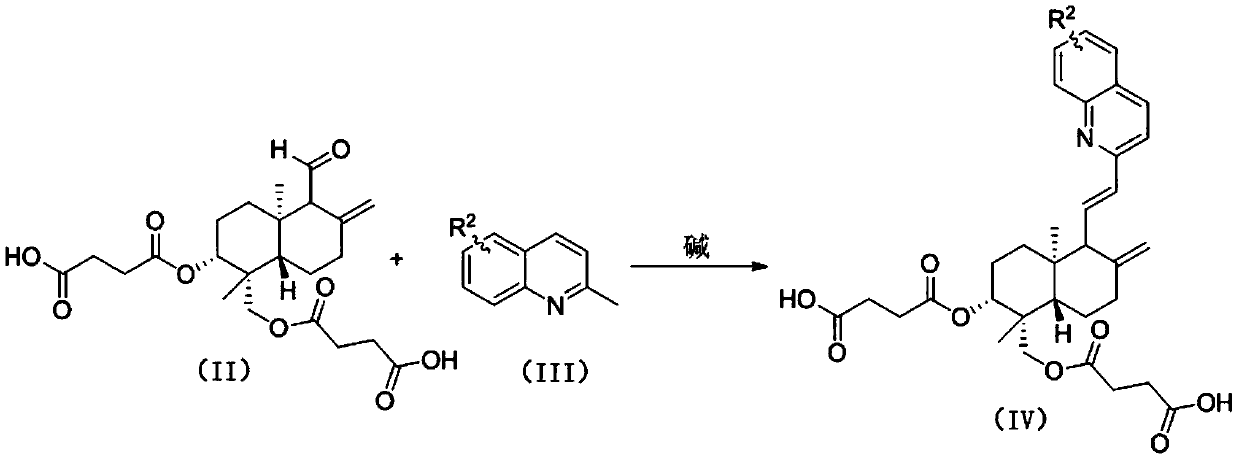
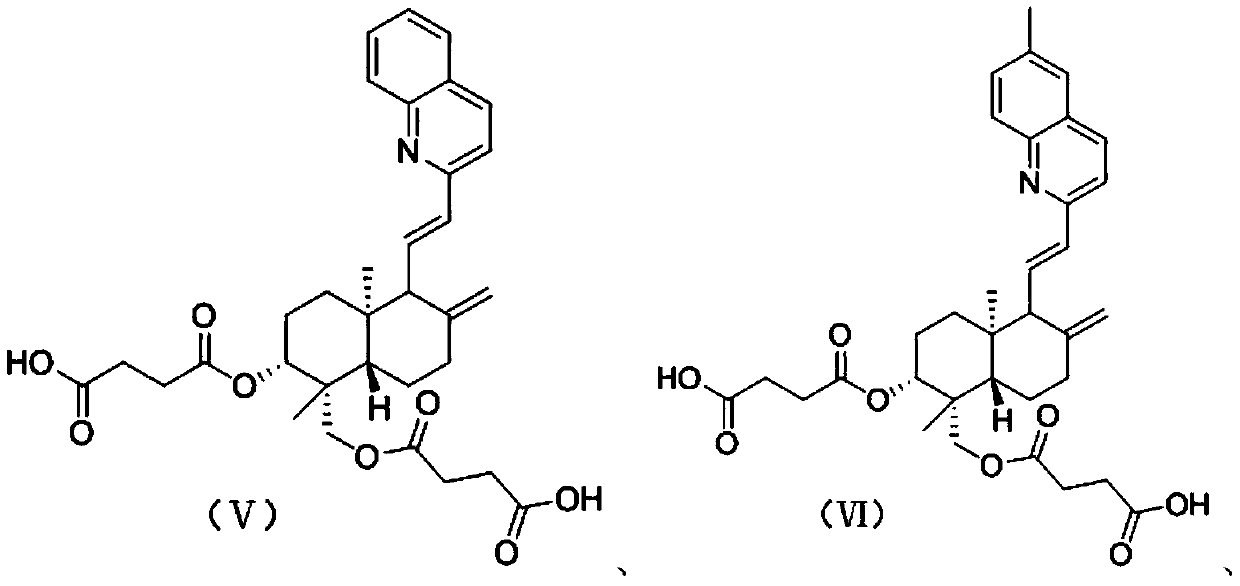
12-quinoline substituted-andrographolide derivative and preparation method and application thereof
A technology of andrographolide and its derivatives, which can be applied in drug combinations, blood diseases, extracellular fluid diseases, etc., and can solve problems such as insufficient anti-platelet aggregation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
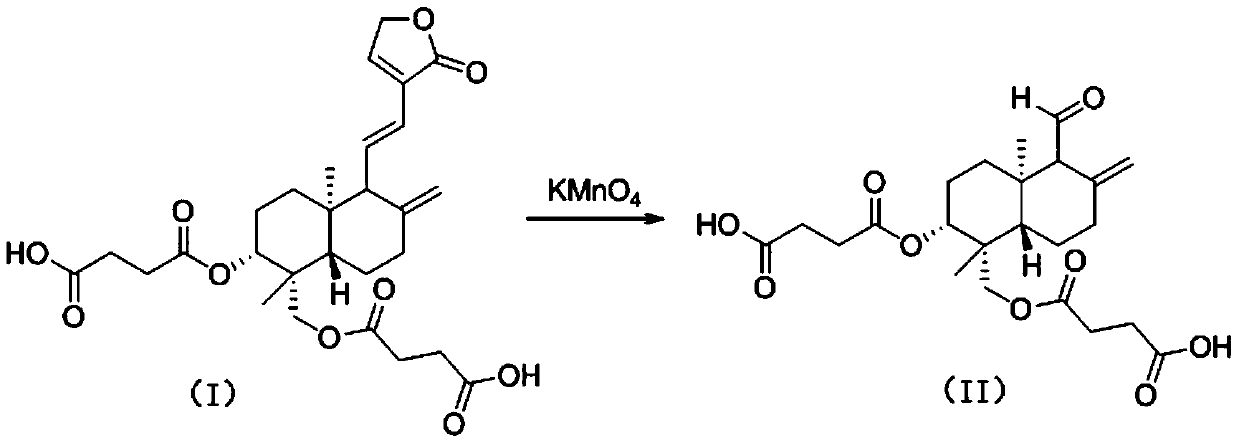
[0026] Preparation of compound (II): in the reaction flask, drop 10g (0.0188mol) of compound (I), add 29.7mL of 10% acidic potassium permanganate solution, the molar ratio is 1:1, then add 40mL of dichloromethane, The reaction was stirred for 1 h at room temperature. Thin-layer chromatography monitoring, after the reaction of the raw materials is complete, concentrate to remove the solvent, then add saturated NaCl aqueous solution, extract three times with dichloromethane, combine dichloromethane, anhydrous NaCl 2 SO 4 After drying and concentrating, 6.644 g of white solid was obtained with a yield of 78.2%.
[0027]
[0028] Compound (II): 1H NMR (400 MHz, DMSO-d6): δ=8.79(s,1H),6.13(s,1H),5.19(m,2H),4.67-4.57(m,1H),4.39– 4.17(m, 2H), 2.77–2.56(m, 7H), 2.48(d, J=2Hz, 1H), 2.47(d, J=12Hz, 1H), 2.34(d, J=10Hz, 1H), 1.83 (dd, J1=1.2Hz, J2=10Hz, 1H), 1.67-1.55(m, 2H), 1.55-1.42(m, 2H), 1.32(dd, J1=2Hz, J2=12Hz, 1H), 1.22- 1.08(m,2H),1.01(s,3H),0.87(s,3H);13C NMR(100 MHz,DM...
Embodiment 2
[0030] The preparation of compound (II): in the reaction flask, drop into 10g (0.0188mol) compound (I), add 59.4mL of 10% acidic potassium permanganate solution, the molar ratio is 1.0:2.0, add 40mL methanol again, at 50 ° C, the reaction was stirred for 24h. Thin-layer chromatography monitoring, after the reaction of the raw materials is complete, cool to room temperature, concentrate to remove the solvent, then add saturated NaCl aqueous solution, extract three times with dichloromethane, combine dichloromethane, anhydrous NaCl 2 SO 4 After drying and concentrating, 6.440 g of white solid was obtained with a yield of 75.8%.
Embodiment 3
[0032] Preparation of compound (II): In the reaction flask, drop 10g (0.0188mol) of compound (I), add 38.6mL of 10% acidic potassium permanganate solution, the molar ratio is 1.0:1.3, and then add 40mL of dimethyl methylene Sulfone, stirred and reacted for 10 h at 80°C. Thin-layer chromatography monitoring, after the reaction of raw materials is complete, cool to room temperature, add 20mL of water, extract three times with dichloromethane, combine dichloromethane, then add saturated NaCl aqueous solution, separate liquid, take dichloromethane layer, anhydrous NaCl 2 SO 4 After drying and concentrating, 6.126 g of white solid was obtained with a yield of 72.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com