Composition for preparing triple compound preparation for treatment of pulmonary tuberculosis and preparation method and application of composition
A technology of compound preparations and compositions, applied in the field of pharmaceutical preparations, which can solve problems such as difficult for patients to swallow, no longer suitable for the treatment process, large tablet size, etc., and achieve the effect of small size, simple preparation process, and easy intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
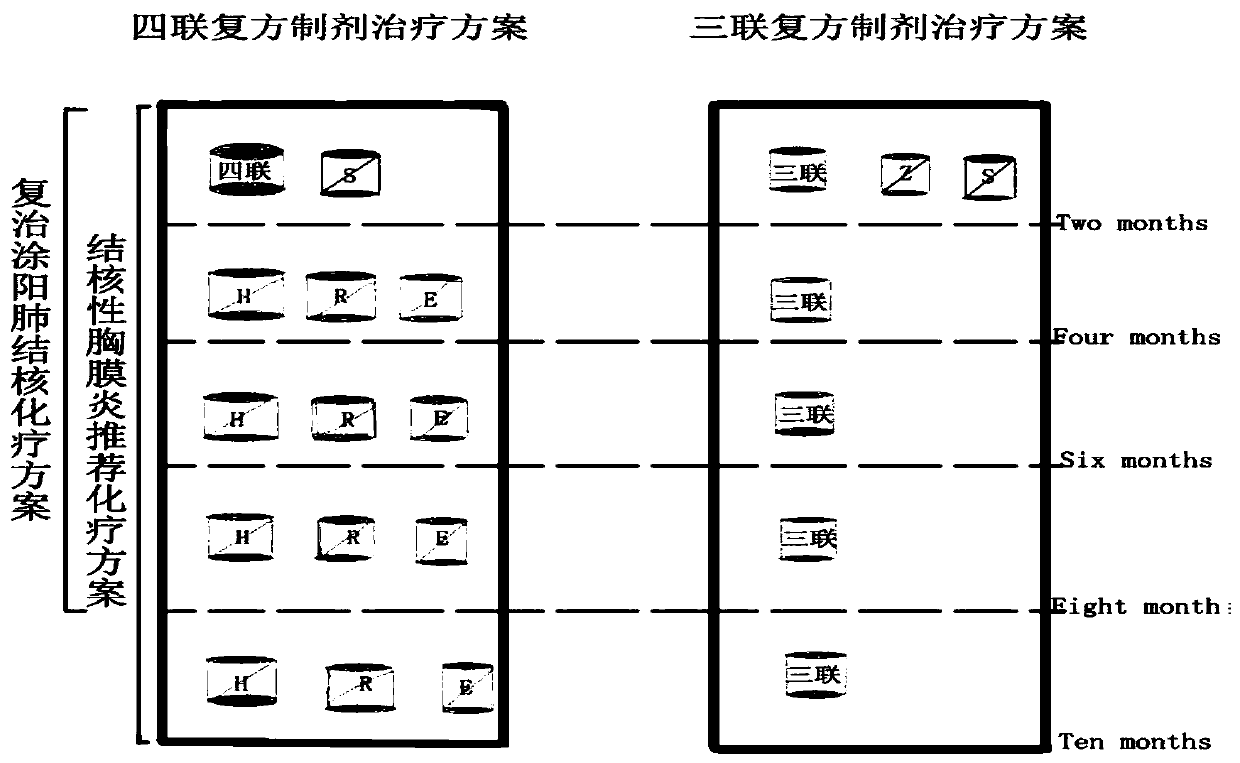
Image
Examples
Embodiment 1
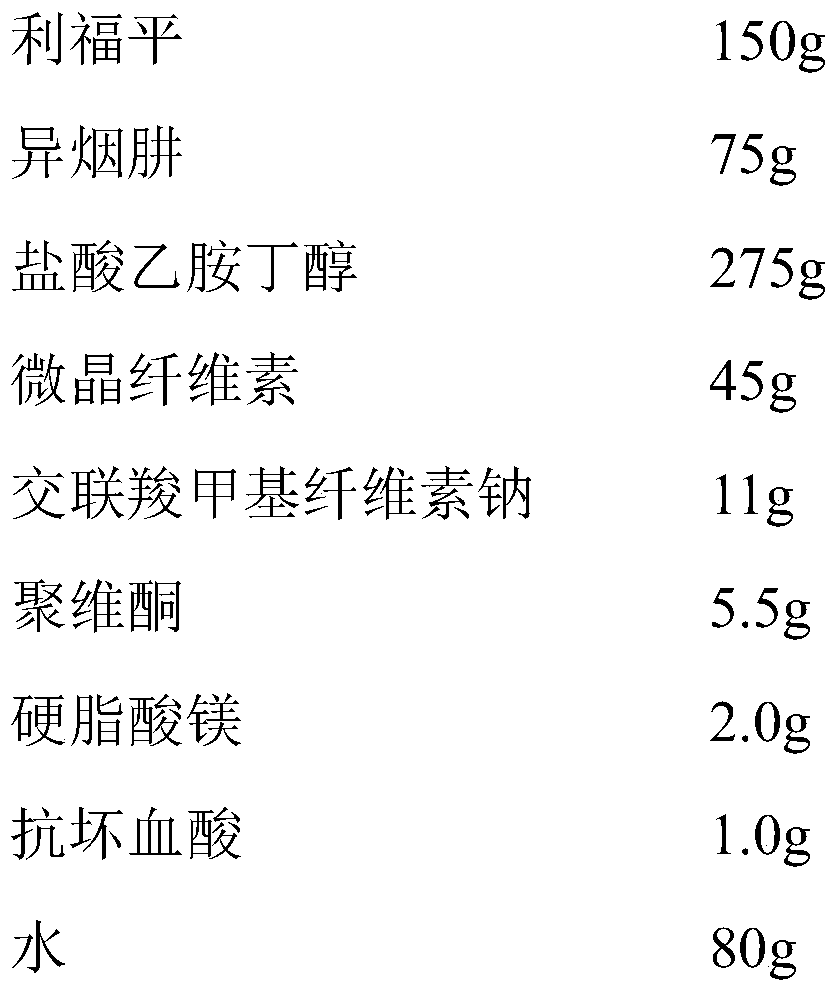
[0057] Example 1 The triple compound preparation for the treatment of pulmonary tuberculosis
[0058] 1. Preparation prescription (made into 1000 tablets)
[0059]
[0060] 2. Preparation process
[0061] 1) Mix isoniazid, ethambutol hydrochloride, microcrystalline cellulose and croscarmellose sodium first, and set aside;
[0062] 2) passing the mixed material through a 60-mesh standard sieve, and dissolving povidone in water to make a solution;
[0063] 3) The granulation parameters are: the rotation speed of the stirring blade is 2 rpm, the rotation speed of the cutter is 25 rpm, and the granulation time is 45 seconds;
[0064] 4) Place the obtained particles in a fluidized bed for drying after passing through a 24-mesh standard sieve;
[0065] 5) The drying parameters are: material temperature: 30-65°C; air inlet temperature: 40-100°C; air volume: 5-40m 3 / h, when the temperature of the material reaches 60-65°C, the material is discharged;
[0066] 4) After the dryi...
Embodiment 2
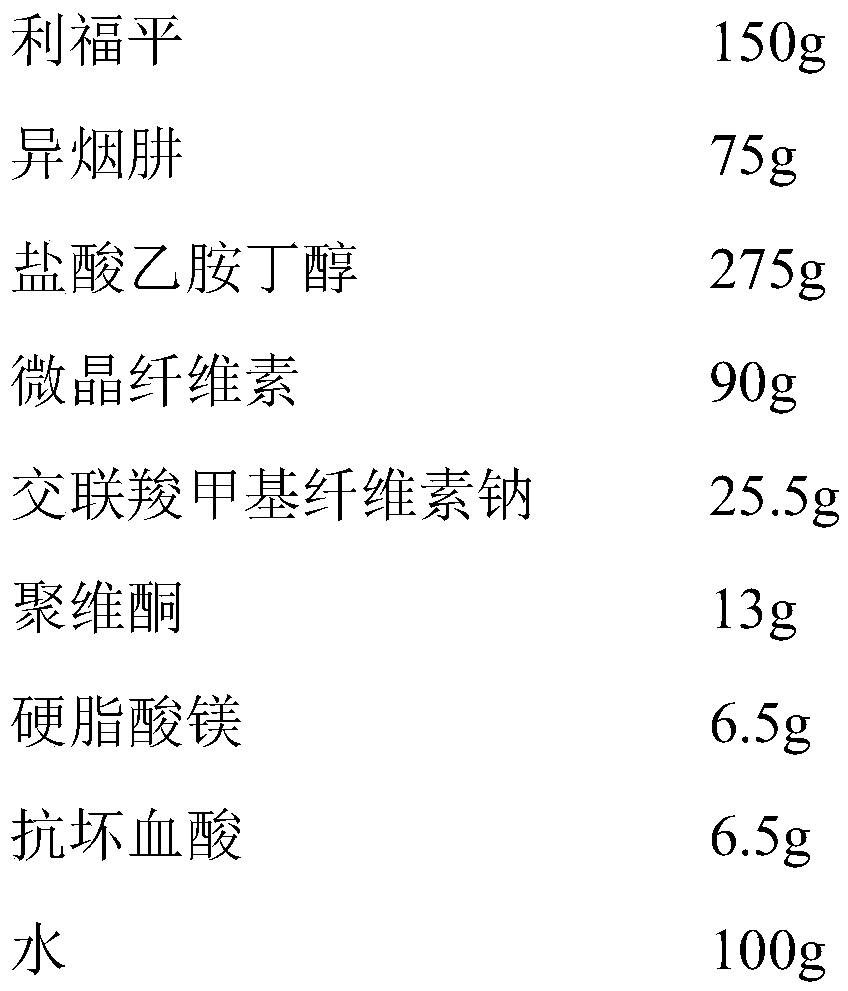
[0068] Example 2 The triple compound preparation for the treatment of pulmonary tuberculosis
[0069] 1. Preparation prescription (made into 1000 tablets)
[0070]
[0071] 2. Preparation process
[0072] 1) mixing isoniazid, ethambutol hydrochloride, microcrystalline cellulose and croscarmellose sodium, and set aside;
[0073] 2) passing the mixed material through a 60-mesh standard sieve, and dissolving povidone in water to make a solution;
[0074] 3) The granulation parameters are: the rotation speed of the stirring blade is 5 rpm, the rotation speed of the cutter is 40 rpm, and the granulation time is 90 seconds;
[0075] 4) Place the obtained particles in a fluidized bed for drying after passing through a 24-mesh standard sieve;
[0076] 5) The drying parameters are: material temperature: 30-65°C; air inlet temperature: 40-100°C; air volume: 10-40m 3 / h, when the temperature of the material reaches 60-65°C, the material is discharged;
[0077] 4) After the drying...
Embodiment 3
[0090] Comparative analysis of quadruple compound preparation and triple compound preparation described in embodiment 3
[0091] The fluidity of the granules, the weight, hardness, friability, and disintegration time of the tablet composition were used as indicators to conduct a comparative study on the formulations of Examples 1-2 and Comparative Example 1.
[0092] 1. Fluidity and compressibility
[0093] Since the Carr index can reflect the fluidity and compressibility of particles, a comparative study was carried out on the above examples. Take an appropriate amount of blended particles, measure them with a powder density meter, and calculate the Carr index.
[0094] Table 1: Carr Index Values of Blend Granules
[0095] Example 1 Example 2 Comparative Example 1 Carr Index (%) 18.4 19.0 27.8
[0096] Note: The calculation method of the Carr index is real density / bulk density*100; the smaller the Carr index, the better the fluidity.
[0097]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com