Rupatadine fumarate emulsion type nasal spray agent and preparation method thereof
A technology of rupatadine fumarate and nasal spray, which is applied in the field of medicine, can solve the problems of large dosage and slow onset of action, and achieve good anti-allergic effects, reduce dosage, and reduce adverse drug reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
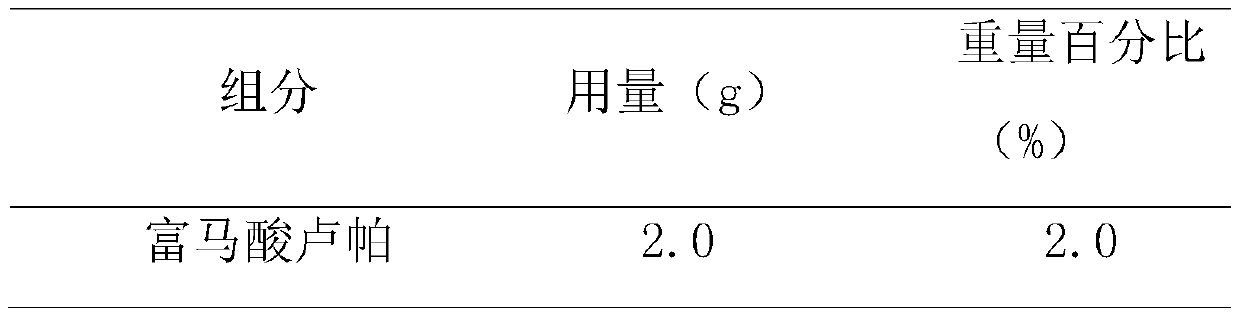
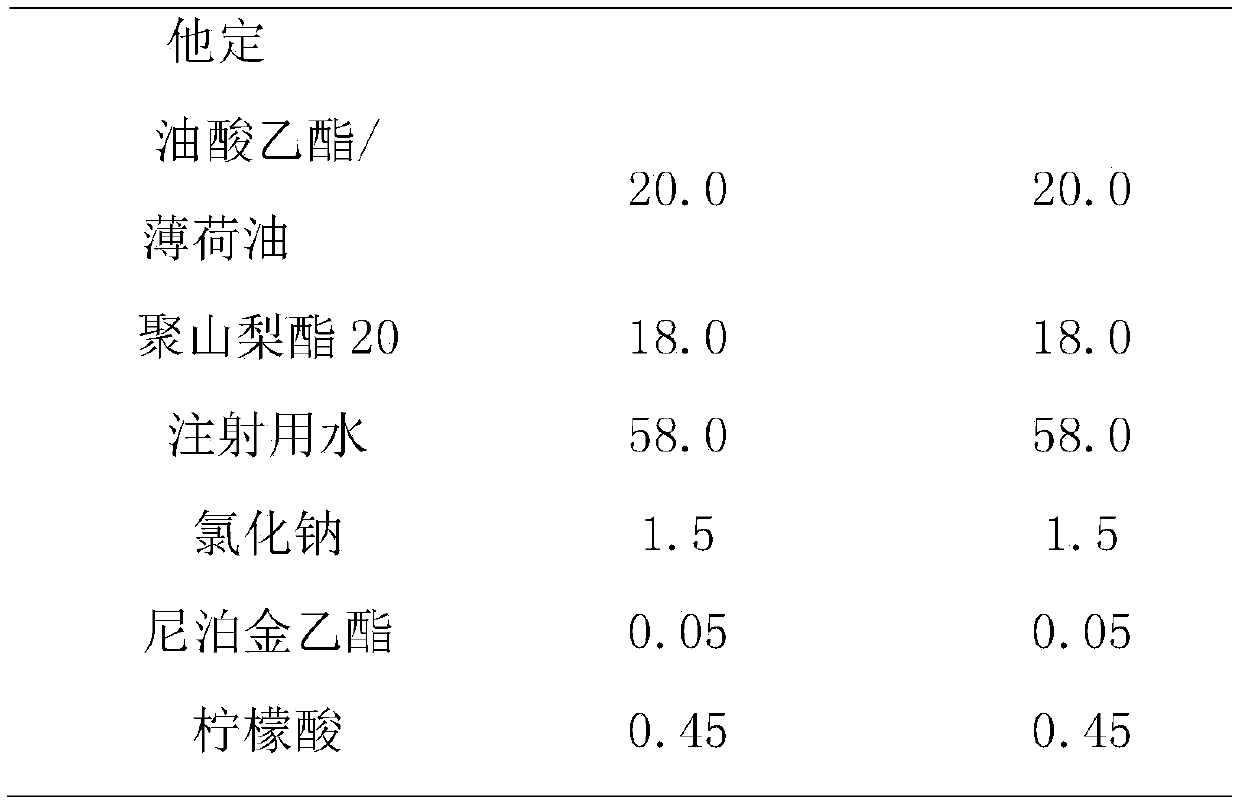
[0024] The prescription composition of rupatadine fumarate emulsion nasal drops is shown in Table 1.
[0025] Table 1. Prescription of Rupatadine Fumarate Emulsion Form Nasal Drops
[0026]
[0027]
[0028] Preparation method of rupatadine fumarate emulsion nasal drops:
[0029] (1) Preparation of oil phase: accurately weigh the recipe quantities of rupatadine fumarate, ethyl oleate / mint oil (10:1, w / w), vortex evenly, and preheat in a 60°C water bath;
[0030] (2) preparation of water phase: accurately weigh the sodium chloride, ethylparaben and polysorbate 20 of the recipe quantity in water for injection, preheat and dissolve in 60 ℃ water bath;
[0031] (3) Preparation of colostrum: adding the oil phase obtained in step (1) to the water phase obtained in step (2), emulsifying with a high-speed shearing homogenizer for 2-5min, 5000-10000rpm;
[0032] (4) Adjust pH value: adjust pH to 5.5-6.5 with citric acid aqueous solution, add water for injection to the recipe am...
Embodiment 2
[0035] The prescription composition of rupatadine fumarate emulsion nasal drops is shown in Table 2.
[0036] Table 2. Prescription of Rupatadine Fumarate Emulsion Form Nasal Drops
[0037]
[0038] Preparation method of rupatadine fumarate emulsion nasal drops:
[0039] (1) Preparation of oil phase: Accurately weigh the recipe quantities of rupatadine fumarate and medium-chain triglyceride, vortex evenly, and preheat in a 60°C water bath;
[0040] (2) preparation of water phase: accurately take by weighing the sodium chloride, sodium benzoate and polyoxyethylene 40 hydrogenated castor oil of the recipe quantity in water for injection, preheat and dissolve in 60 ℃ water bath;
[0041] (3) Preparation of colostrum: adding the oil phase obtained in step (1) to the water phase obtained in step (2), emulsifying with a high-speed shearing homogenizer for 2-5min, 5000-10000rpm;
[0042] (4) Adjust pH value: adjust pH to 5.5-6.5 with citric acid aqueous solution, add water for i...
Embodiment 3
[0045] The prescription composition of rupatadine fumarate emulsion nasal drops is shown in Table 3.
[0046] Table 3. Recipe of Rupatadine Fumarate Emulsion Nasal Drops
[0047]
[0048] Preparation method of rupatadine fumarate emulsion nasal drops:
[0049] (1) Preparation of oil phase: Accurately weigh the recipe quantities of rupatadine fumarate and castor oil, vortex evenly, and preheat in a 60°C water bath;
[0050] (2) Preparation of aqueous phase: accurately weigh the sodium chloride, ethylparaben and poloxamer 188 of the recipe in water for injection, preheat and dissolve in a 60°C water bath;
[0051] (3) Preparation of colostrum: adding the oil phase obtained in step (1) to the water phase obtained in step (2), emulsifying with a high-speed shearing homogenizer for 2-5min, 5000-10000rpm;
[0052] (4) Adjust pH value: adjust pH to 5.5-6.5 with citric acid aqueous solution, add water for injection to the recipe amount, and filter;
[0053] (5) Sterilization and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com