Oleracone H in purslane, extraction and separation method thereof, and application thereof
A separation method, the technology of purslane, applied in the direction of organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problem of low structural novelty, and achieve the effect of environmentally friendly process method, simple and fast operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention provides new compound, molecular formula is C 13 h 14 o 6 , named oleracone H chemical formula is: .
[0031] The new compound is named oleracone H according to the structure, and Table 1 is the NMR data of the new compound: 1 H-NMR with 13 C-NMR in deuterated DMSO.
[0032] Table 1 NMR data of the new compound ( 1 H-NMR with 13 C-NMR in deuterated DMSO).
[0033]
[0034] The structural identification of the compounds of the present invention can be referred to Figure 1-10 .
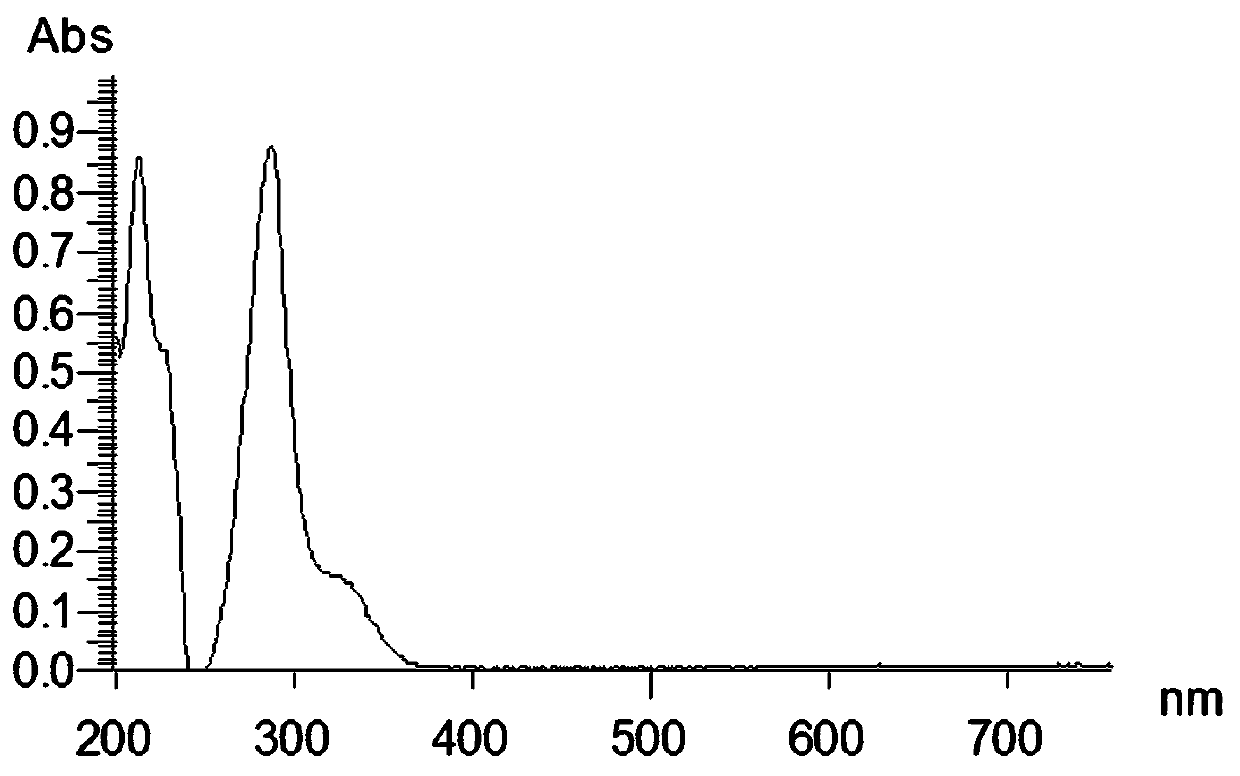
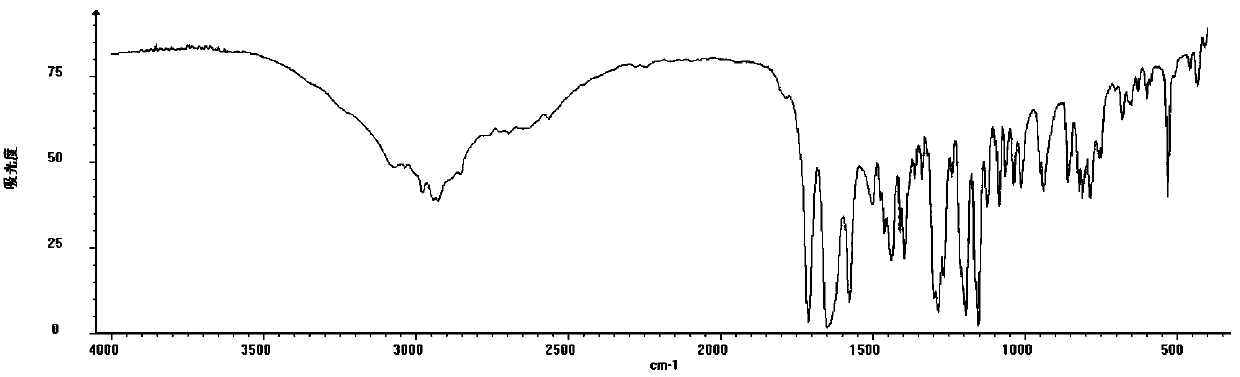
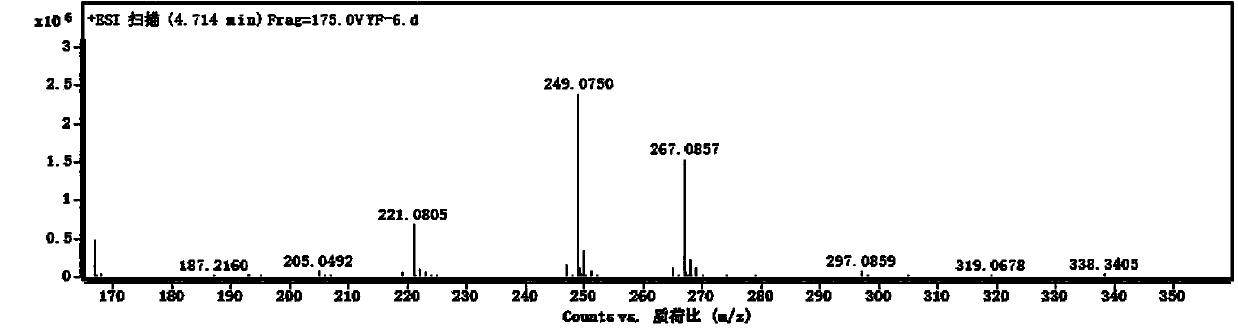
[0035] Oleracone H: Yellow-green powder, easily soluble in methanol. After spotting the sample on the silica gel thin-layer plate, spray the ferric chloride test solution and the spot turns blue. UV(MeOH)λ max : 288, 213 nm. IR (KBr) v max 2940, 1710, 1650, 1575, 1280, 1150, 1125, 940, 856, 815, 784 cm -1 . HRESI(+)TOFMS gives m / z: 267.0857 [M+H] + The quasi-molecular ion peak has a molecular weight of 267.0864. combine 1 H-NMR, 13 According to C-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com