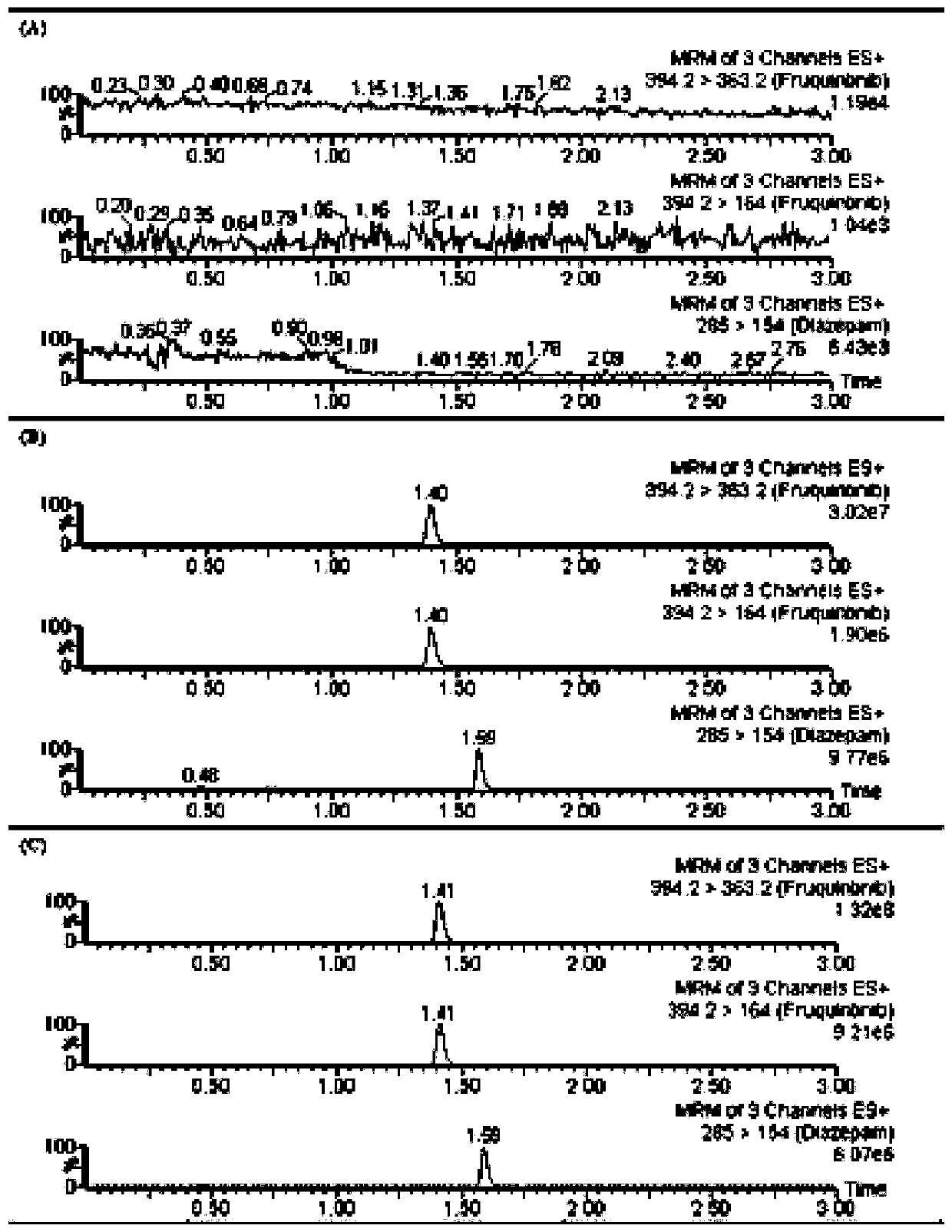
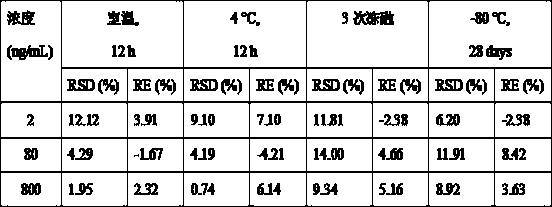
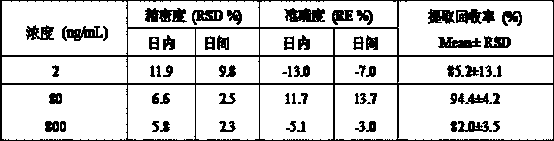
UPLC-MS/MS (Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry) method for rapidly detecting fruquintinib in rat plasma
A fruquintinib and plasma technology, applied in the field of UPLC-MS/MS for rapid detection of fruquintinib in rat plasma, can solve problems such as inability to repeat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Specimen collection: Fruquintinib was dissolved in a 0.4% sodium carboxymethyl cellulose solution at a concentration of 0.8 mg / kg and administered orally to a group of young male rats. For h, 1h, 1.5h, 2h, 3h, 4h, 6h, 9h, 12h, 24h and 48h, blood samples of 0.25ml were collected from the tail vein of rats, respectively. After centrifugation at 3000 rpm for 4min, 90µL of supernatant plasma was taken and stored in -20 ℃ refrigerator for testing.
[0020] 2) Plasma sample processing: 0.08 mL of plasma sample was directly precipitated with 180 µL of acetonitrile, in which the internal standard substance diazepam was directly dissolved in acetonitrile, and the diazepam concentration was 90 ng / mL; after vortexing for 0.8 min, 12000 Centrifuge at rpm for 8 min, and take 5 µL of the supernatant for mass spectrometry detection.
[0021] 3) Mass spectrometry detection: Detection by Acquity UPLC BEH C18 chromatographic column, the column temperature is 39 °C, the mobile phase c...
Embodiment 2
[0024] 1) Specimen collection: Fruquintinib was dissolved in a 0.5% sodium carboxymethyl cellulose solution at a concentration of 1.0 mg / kg and administered orally to a group of young male rats. After h, 1h, 1.5h, 2h, 3h, 4h, 6h, 9h, 12h, 24h and 48h, 0.3ml blood samples were collected from the tail vein of rats, respectively. After centrifugation at 1000 rpm for 5min, 100µL of supernatant plasma was taken and stored in -20℃ refrigerator for testing;
[0025] 2) Plasma sample processing: 0.1 mL of plasma sample was directly precipitated with 200 µL of acetonitrile. The internal standard substance, diazepam, was directly dissolved in acetonitrile, and the concentration of diazepam was 100 ng / mL; Centrifuge for 10 min, and take 6 µL of the supernatant for mass spectrometry detection;
[0026] 3) Mass spectrometry detection: detected by Acquity UPLC BEH C18 chromatographic column, the column temperature was 40 °C, the mobile phase consisting of acetonitrile and 0.1% formic acid ...
Embodiment 3
[0029] 1) Specimen collection: Fruquintinib was dissolved in a 0.6% sodium carboxymethyl cellulose solution at a concentration of 1.2 mg / kg and administered orally to a group of young male rats. After h, 1h, 1.5h, 2h, 3h, 4h, 6h, 9h, 12h, 24h and 48h, blood samples of 0.35ml were collected from the tail vein of rats, respectively. After centrifugation at 5000 rpm for 6min, 110µL of supernatant plasma was taken and stored in -25℃ refrigerator for testing;
[0030] 2) Plasma sample processing: 0.11mL plasma sample was directly precipitated with 220µL acetonitrile, in which the internal standard substance diazepam was directly dissolved in acetonitrile, and the diazepam concentration was 110 ng / mL; after vortexing for 1.2min, 14000 Centrifuge at rpm for 12 min, and take 7 µL of the supernatant for mass spectrometry detection;
[0031]3) Mass spectrometry detection: detection by Acquity UPLC BEH C18 chromatographic column, the column temperature is 41 °C, the mobile phase consist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com