Combined detection kit and detection method for viruses associated with respiratory infection nucleic acid
A joint detection and viral nucleic acid technology, applied in the biological field, can solve the problems of joint detection of respiratory syncytial virus without influenza A virus and influenza B virus, and achieve the effects of convenient new drug development, pollution reduction, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 A kind of respiratory virus nucleic acid combined detection kit
[0079] (1) Extraction of respiratory virus nucleic acid
[0080] The nucleic acid extraction and purification reagents produced by Shanghai Fosun Changzheng Medical Science Co., Ltd. were used to extract nucleic acid from human nasopharyngeal swab samples.
[0081] (2) Fluorescent RT-PCR amplification
[0082] a. Add sample:
[0083] Add 20 μL of nucleic acid extraction solution to the detection tube containing 30 μL of reaction solution, and the reaction volume is 50 μL;
[0084] b. One-step RT-PCR reaction procedure:
[0085]
[0086] c. Collect fluorescence signals of FAM, HEX, ROX and CY5 channels in the eighth step.
[0087] (3) Detection: the present invention is applicable to ABI 7500 fluorescent quantitative PCR instrument for virus nucleic acid identification and detection.
[0088] (4) Result judgment:
[0089] A. Quality control:
[0090] The Ct values detected by the C...
Embodiment 2
[0097] Embodiment 2 clinical detection
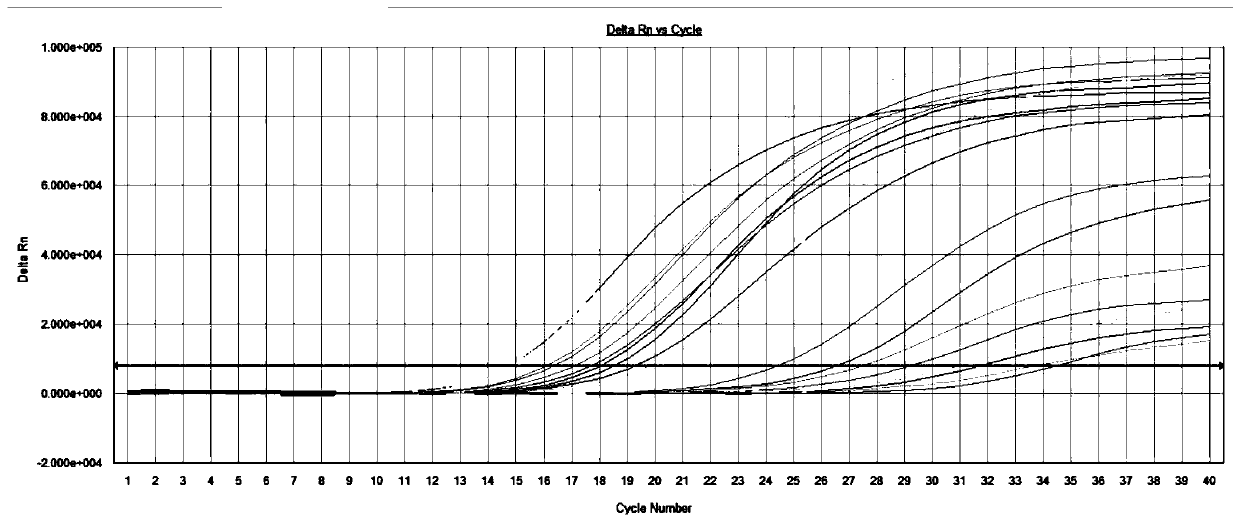
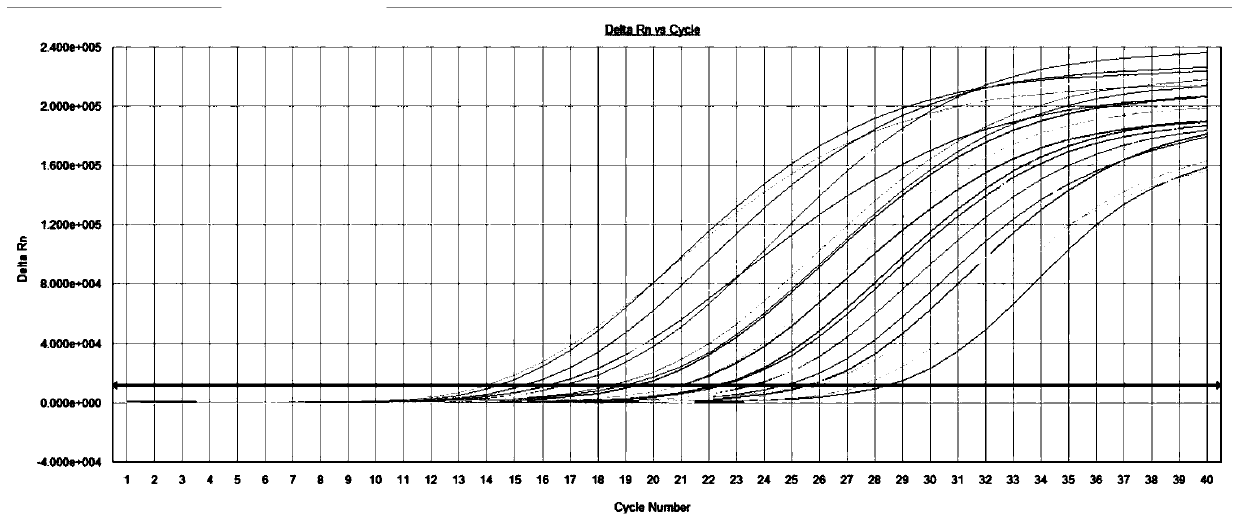
[0098] Using the method above, 74 cases of clinical samples were qualitatively detected by fluorescent PCR, including 18 patients with influenza A virus (including 1 patient who was positive for both influenza A virus and respiratory syncytial virus). figure 1 16 cases of influenza A virus patients were detected by the colloidal gold detection method commonly used in clinical practice; the influenza A / B nucleic acid detection kit (fluorescent PCR method) produced by Shuoshi Biotechnology Co., Ltd., which has been listed, (Cat. No. : JC10202N) The fluorescent PCR confirmation test was performed on the 2 samples with inconsistent detection results by the two methods: both samples were positive for influenza A virus (see figure 2 ), showing that the detection efficiency of the inventive method is high; 21 cases of patients with influenza B virus, the fluorescence detection results are shown in image 3 100% (21 / 21) coincidence rate with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com