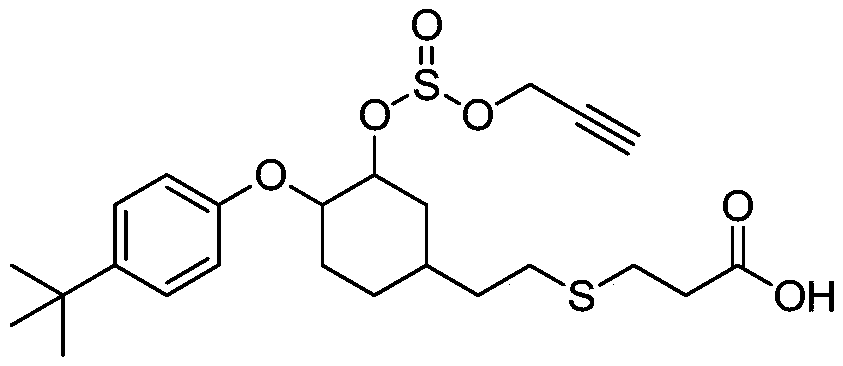
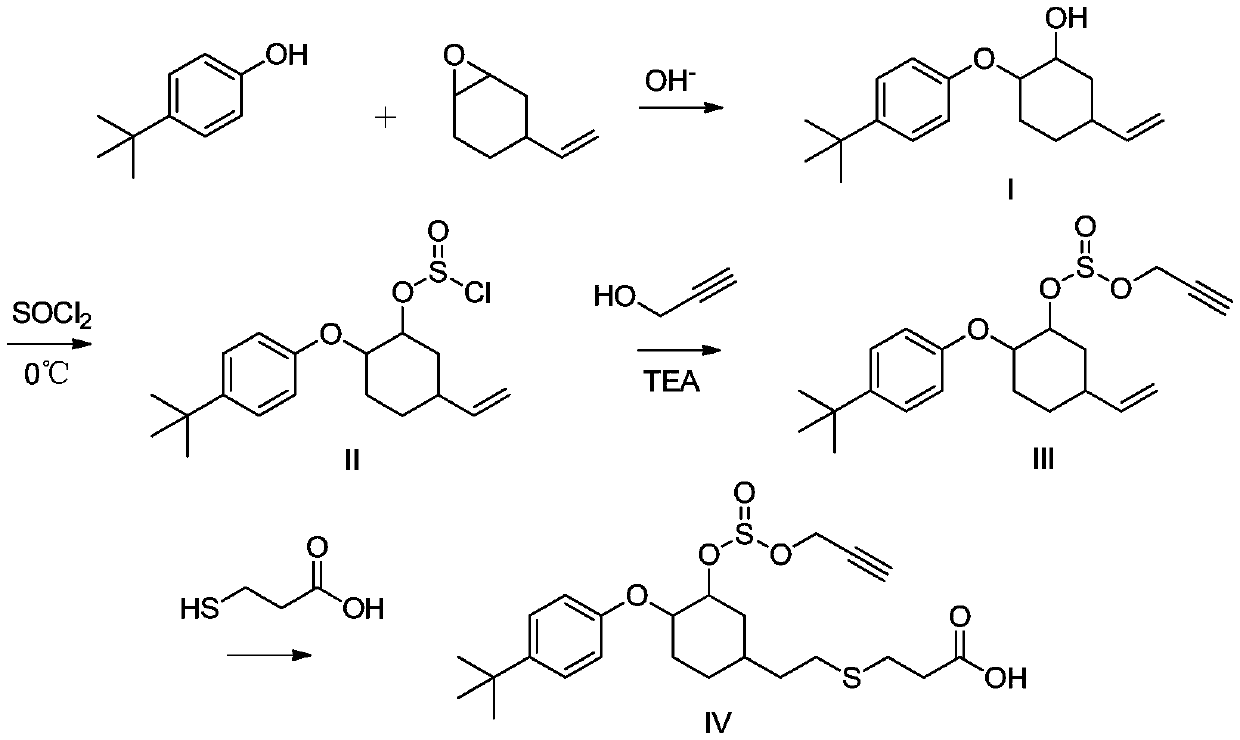
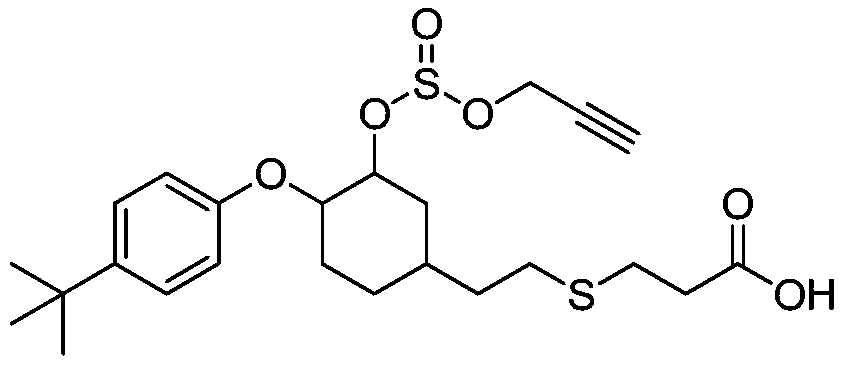
Propargite hapten and synthesis method and application thereof
A synthesis method and technology of propargite, which are applied in the field of hapten and synthesis of propargite, achieve the effects of simple experimental operation, high purity and yield, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A method for preparing a propargite hapten, comprising the steps of:
[0039] (1) 4-tert-butylphenol and 1,2-epoxy-4-vinylcyclohexane react to prepare intermediate product I:
[0040] Add 4-tert-butylphenol (1.00g, 6.66mmol) and 1,2-epoxy-4-vinylcyclohexane (1.00g, 8.00mmol) successively to a 50ml single-necked bottle, stir at room temperature until dissolved; Add potassium hydroxide (75mg, 1.33mmol) and toluene (1.00ml), raise the temperature to 105°C and stir for 18h; drop to room temperature and stir, add toluene (20ml), wash with 5% sodium hydroxide solution (60ml) in three equal portions, Get the organic layer; the organic layer is dried with anhydrous sodium sulfate, filtered, and the filtrate is taken; the filtrate column chromatography, the eluent is petroleum ether: ethyl acetate, and the volume ratio is 10:1; the target liquid is separated, and the solvent is removed by distillation. 1.7 g of a colorless transparent oily substance was obtained, which was the ...
Embodiment 2
[0048] A method for preparing a propargite hapten, comprising the steps of:
[0049] (1) 4-tert-butylphenol and 1,2-epoxy-4-vinylcyclohexane react to prepare intermediate product I:
[0050] Add sodium hydroxide (350mg, 8.66mmol) and purified water (13.00ml) to a 50ml single-necked bottle in turn, stir at room temperature until it dissolves; add 4-tert-butylphenol (1.00g, 6.66mmol), stir at room temperature until it dissolves Add 1,2-epoxy-4-vinylcyclohexane (1.00g, 8.00mmol) dropwise in 3 to 5 minutes, stir at room temperature until dissolved, and react at room temperature for 18 hours; filter the reaction solution, and filter the cake with purified water ( 40ml) was washed twice, and then washed with n-hexane (20ml), and the obtained filter cake was dried at room temperature; 1.65g of white solid was intermediate product I, yield: 90.3%.
[0051] (2) intermediate product I reacts with sulfur oxychloride to prepare intermediate product II
[0052] Add toluene (1.10ml) and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com