Method for synthesizing n-thiobenzyl imine or its derivatives by bunte salt
A technology of thiobenzyl imine and derivatives, which is applied in the field of organic synthesis, can solve the problems of large environmental hazards, small substrate range, and raw material restrictions, and achieve the effects of reasonable process conditions, lower reaction temperature, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Add 0.0428g (0.4mmol) benzylamine, 0.09g (0.6mmol) sodium methylthiosulfate, 0.0574g (0.4mmol) cuprous bromide and 2mL (25mmol) N,N- dimethylformamide. The reaction was stirred at 80°C for 10h. After the reaction, the reaction solution was diluted with 20 mL of water, extracted three times with 15 mL of ethyl acetate, the organic phases were combined, washed with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, the volume ratio of which was 50:1) to obtain 0.0423 g of N-methylthiobenzyl imine with a yield of 70%.
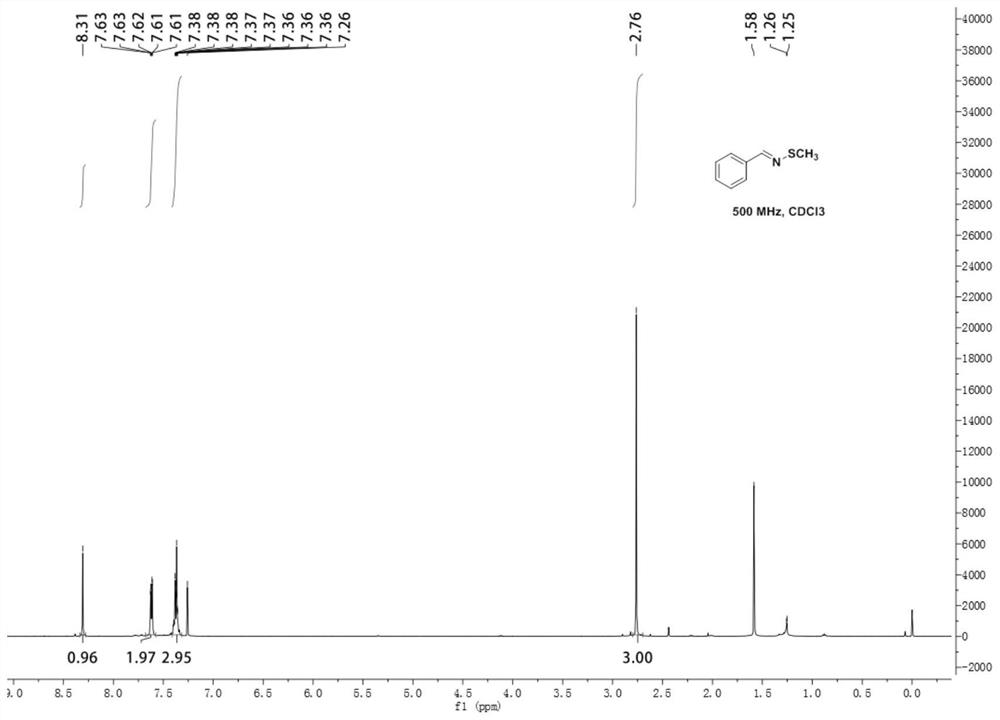
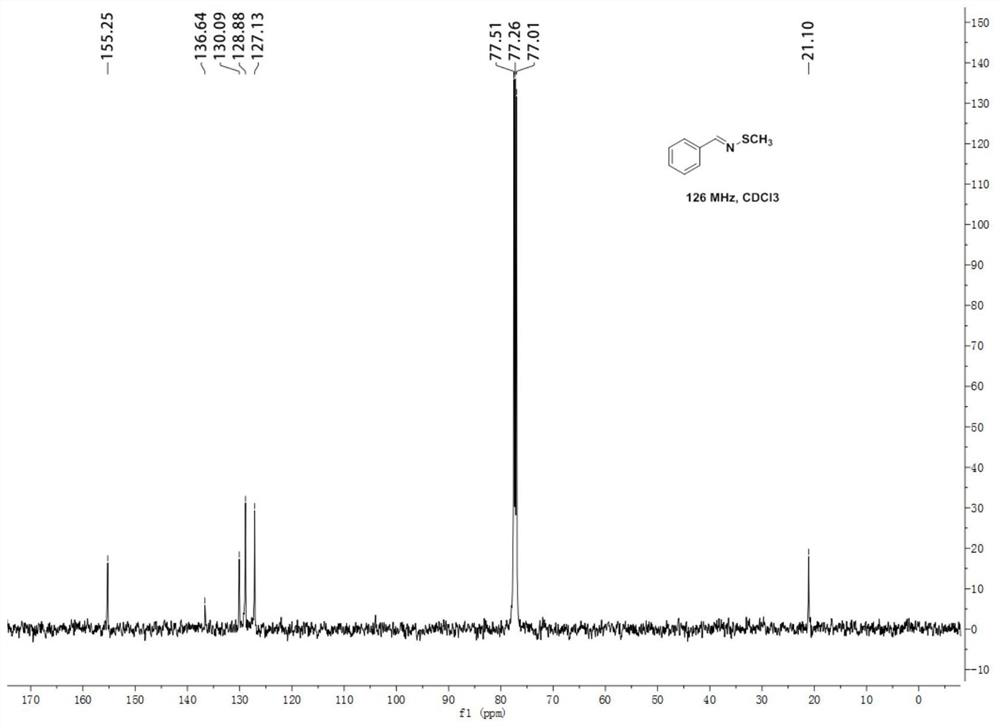
[0048] N-methylthiobenzyl imine 1 H NMR diagram see figure 1 , N-Methylthiobenzimine 13 C NMR chart see figure 2 .
[0049] 1 H NMR (500MHz, Chloroform-d) δ8.31(s, 1H), 7.62(dd, J=7.8, 1.9Hz, 2H), 7.37(td, J=5.9, 2.8Hz, 3H), 2.76(s, 3H); ...
Embodiment 2
[0052] Add 0.0744g (0.4mmol) p-bromobenzylamine, 0.09g (0.6mmol) sodium methylthiosulfate, 0.0574g (0.4mmol) cuprous bromide and 2mL (25mmol) N to a 35mL thick-walled pressure-resistant tube, N-Dimethylformamide. The reaction was stirred at 80°C for 10h. After the reaction, the reaction solution was diluted with 20 mL of water, extracted three times with 15 mL of ethyl acetate, the organic phases were combined, washed with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, the volume ratio of which was 30:1) to obtain 0.0614 g of N-methylthio-4-bromobenzyl imine with a yield of 67 %.
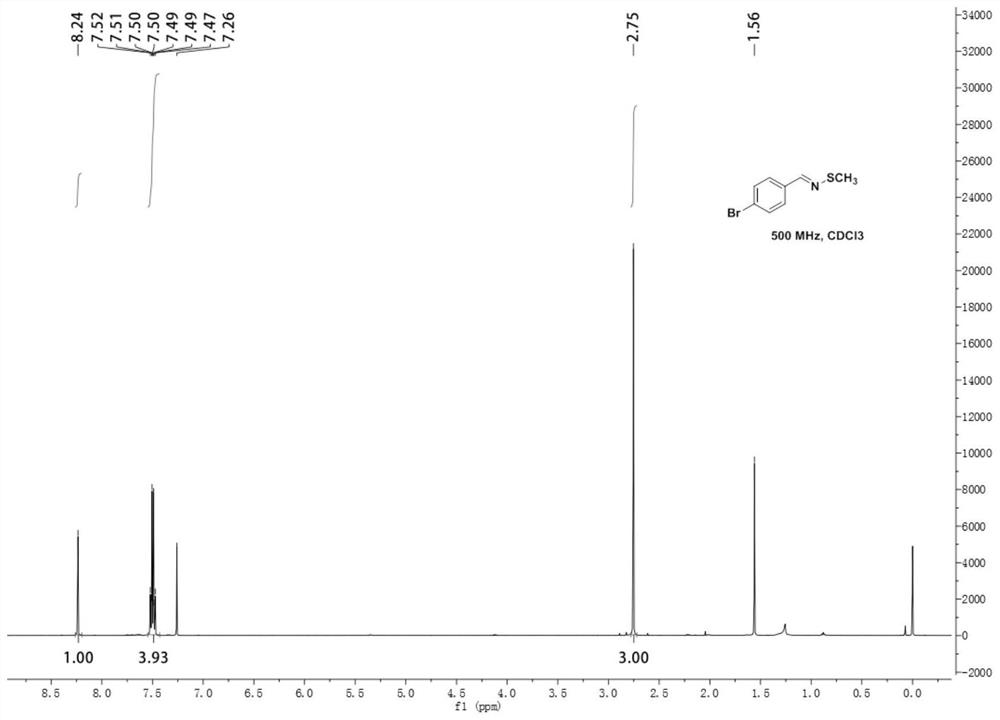
[0053] N-methylthio-4-bromobenzyl imine 1 H NMR diagram see image 3 , N-methylthio-4-bromobenzimine 13 C NMR diagram see Figure 4 .
[0054] 1 H NMR (500MHz, Chloroform-d) δ...
Embodiment 3
[0057] Add 0.07g (0.4mmol) 4-trifluoromethylbenzylamine, 0.09g (0.6mmol) sodium methylthiosulfate, 0.0574g (0.4mmol) cuprous bromide and 2mL ( 25mmol) N,N-dimethylformamide. The reaction was stirred at 80°C for 10h. After the reaction, the reaction solution was diluted with 20 mL of water, extracted three times with 15 mL of ethyl acetate, the organic phases were combined, washed with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, and the volume ratio of the two was 10:1) to obtain 0.0569 g of N-methylthio-4-trifluoromethylbenzyl imine, producing The rate is 65%.
[0058] N-Methylthio-4-trifluoromethylbenzyl imine 1 H NMR diagram see Figure 5 , N-methylthio-4-trifluoromethylbenzyl imine 13 C NMR diagram see Image 6 , N-methylthio-4-trifluoromethylbenzyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com