Application of oridonin in preparing drugs for preventing or treating NLRP3 inflammasome-related diseases
A technology of oridonin A and inflammasome is applied in metabolic diseases, skin diseases, bone diseases and other directions, which can solve the problems of unknown side effects, insufficient direct action mechanism, unclear application prospects, etc., and achieve strong medicinal prospects and pharmacological effects. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
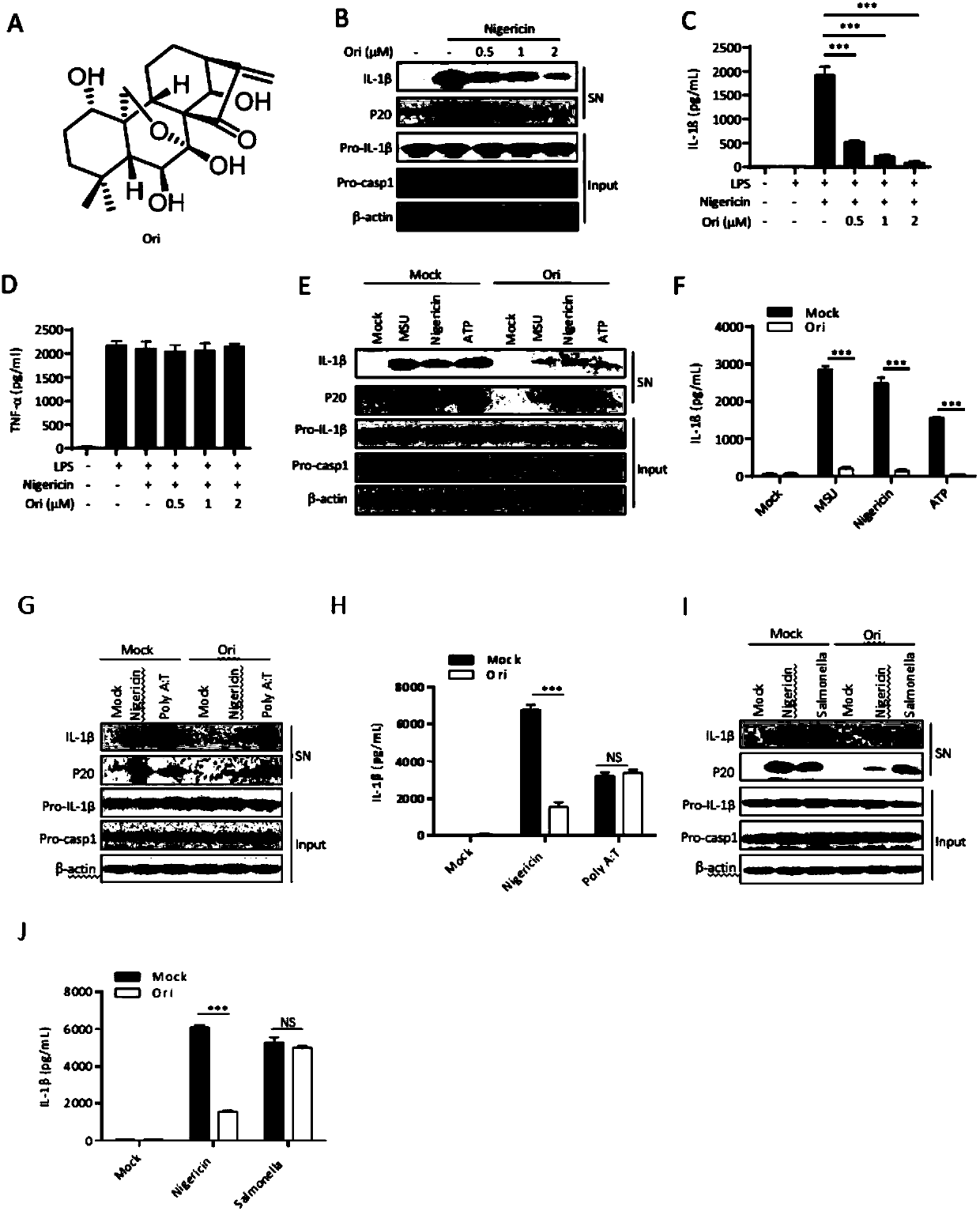
[0128] Example 1 In vitro study on the inhibitory effect of oridonin on NLRP3 inflammasome
[0129] 1. Experimental method
[0130] 1. Collection of L-929 cell culture supernatant
[0131] L-929 cells were cultured in a 15 cm treated culture dish (purchased from Fisher). After the cells were confluent, the culture supernatant was collected, centrifuged to remove dead cells, and then filtered to remove impurities, and stored in a -20°C refrigerator for later use.
[0132] 2. Acquisition of BMDM cells (bone marrow-derived macrophages)
[0133] (1) Six-week-old wild-type C57BL / 6J mice were taken, sacrificed by cervical dislocation, and hindlimb leg bones were taken.
[0134] (2) In the ultra-clean bench, the bone marrow in the leg bones of the hind legs was blown out and centrifuged.
[0135] (3) The supernatant was discarded, and the erythrocytes were lysed, then neutralized with DMEM medium (purchased from HyClone), and centrifuged.
[0136] (4) Discard the supernatant, add...
Embodiment 2
[0173] Example 2 Mechanism of Rubescensine A Inhibiting NLRP3 Inflammasome Activation
[0174] 1. Experimental method
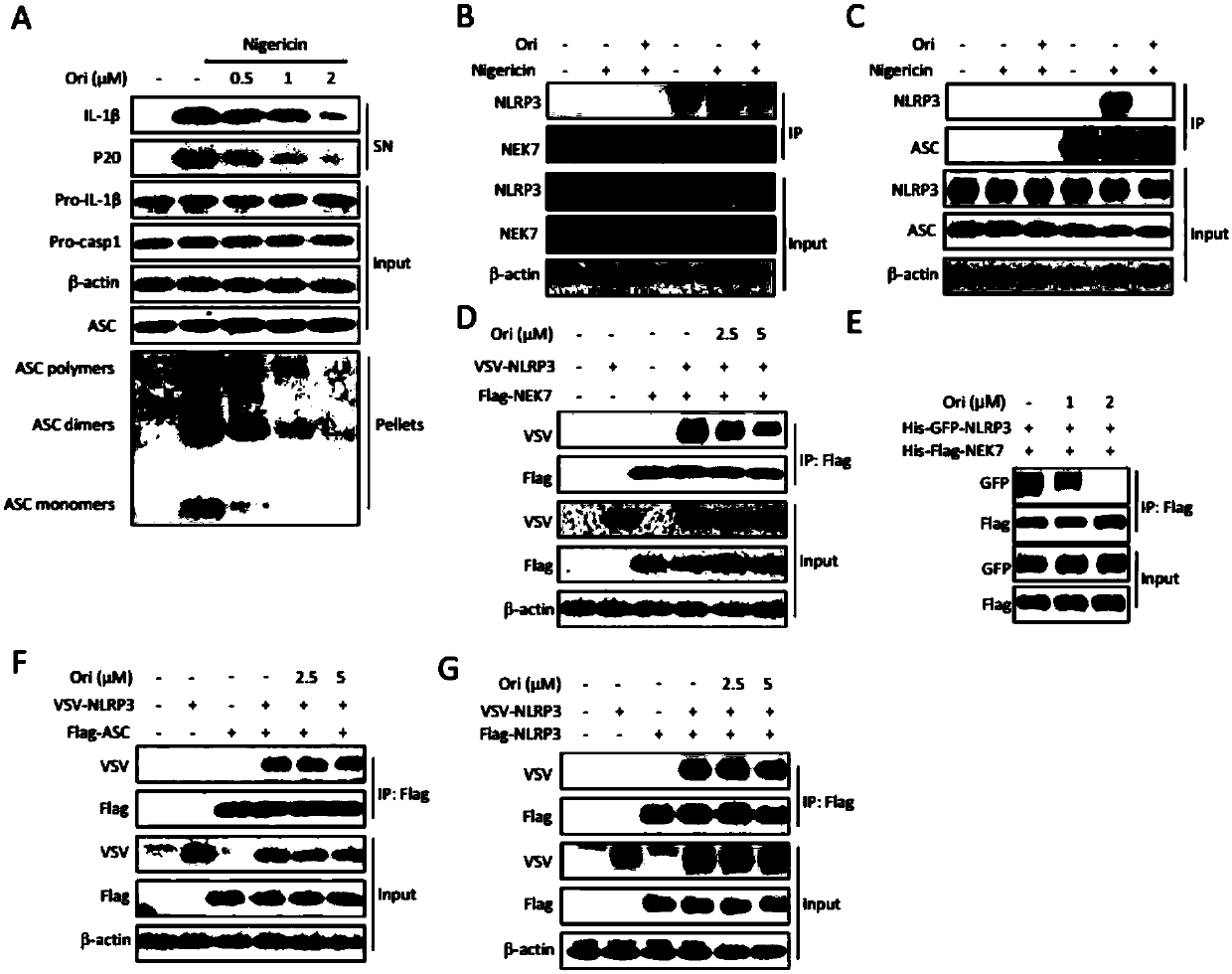
[0175] 1. ASC (apoptosis-associated speck-like protein) aggregation experiment
[0176] (1) Divide the differentiated cells into 6-well plates, carry out normal inflammasome stimulation, collect supernatant protein for WB, then wash the cells three times with pre-cooled PBS, add 300ul pre-cooled NP40 (purchased from Bi Yuntian), lysed on a shaker at 4°C for 0.5 hours.
[0177] (2) Centrifuge at 330g at 4°C for 10 minutes, discard the supernatant, wash three times with pre-cooled PBS, and resuspend with 500 μl PBS.
[0178] (3) DSS (disuccinimide suberate, purchased from Sanko) was added to a final concentration of 2 mM, and then rotated at room temperature for 30 minutes.
[0179] (4) Centrifuge at 330g for 10 minutes, discard the supernatant, add sample buffer, 101°C metal bath, 10 minutes, load the sample or store at -20°C for later use.
[0180] 2. End...
Embodiment 3
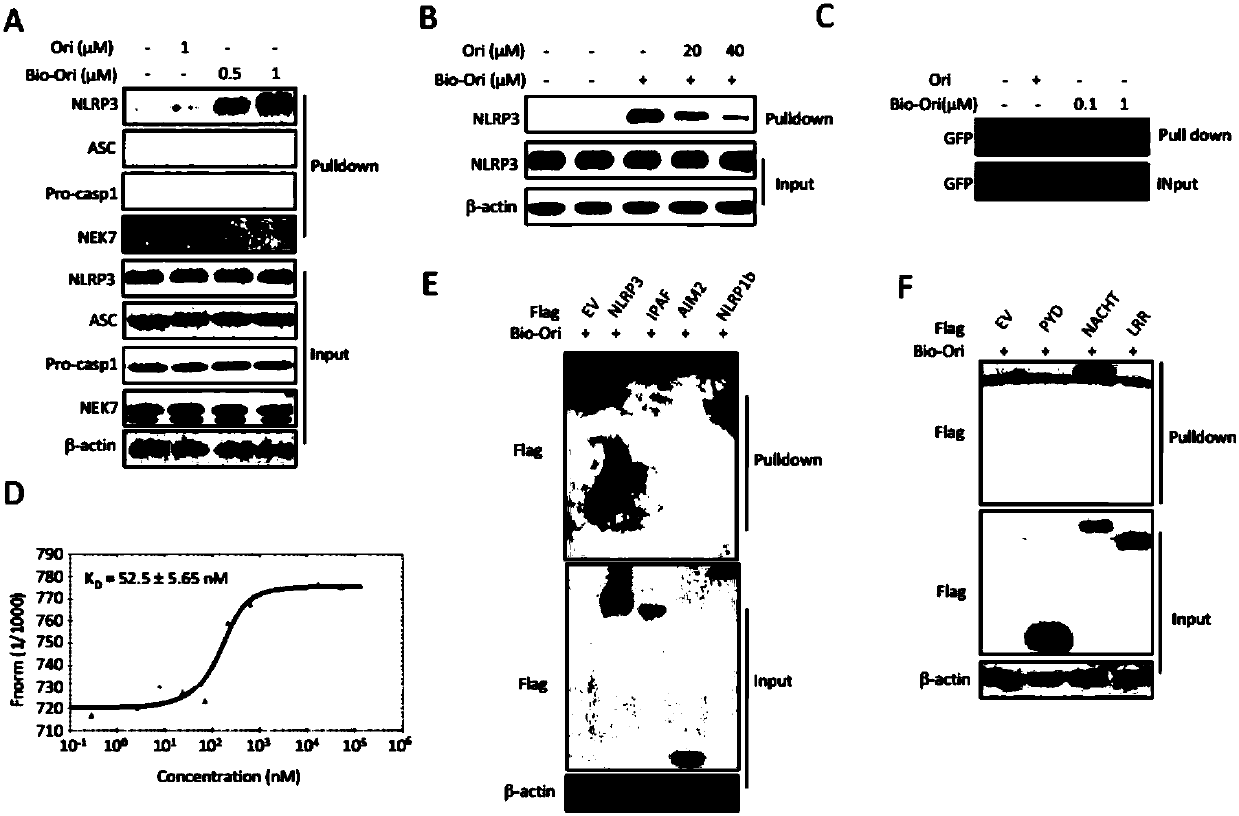
[0217] Example 3 Research on the Binding Mode and Binding Site of Oridonin A and NLRP3
[0218] 1. Experimental method
[0219] 1. Elution experiment
[0220] Divide BMDM cells into 12-well plates, add LPS to stimulate for 3 hours the next day, and then add Rubescensin A. After adding Rubescensin A for 15 minutes in the elution group, use the medium without Rubescensin A Replacement was performed, repeated after 5 minutes, and eluted 3 times in total, and then normal inflammasome stimulation samples were collected for ELISA.
[0221] 2. Stimulation of inflammasomes, same as in Example 1.
[0222] 3. Biotin Pull Down (protein binding experiment in vitro), same as Example 2.
[0223] 4. Exogenous co-immunoprecipitation is the same as in Example 2.
[0224] 2. Results Analysis
[0225] The binding between protein and small molecule has covalent bond and non-covalent bond. After the elution of oridonin, it can still inhibit the activation of inflammasomes ( Figure 4 A), wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com