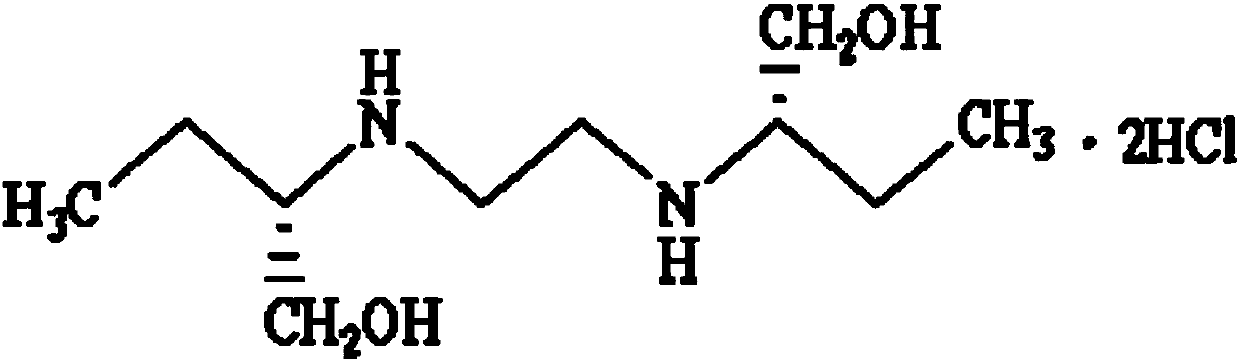
Preparation method for ethambutol hydrochloride
A technology for ethambutol hydrochloride and ethambutol, which is applied in the field of pharmaceutical preparation technology and chemistry, can solve the problems of high production cost, difficult disposal of waste liquid, too many kinds of raw materials, etc., and achieves the effects of low cost and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
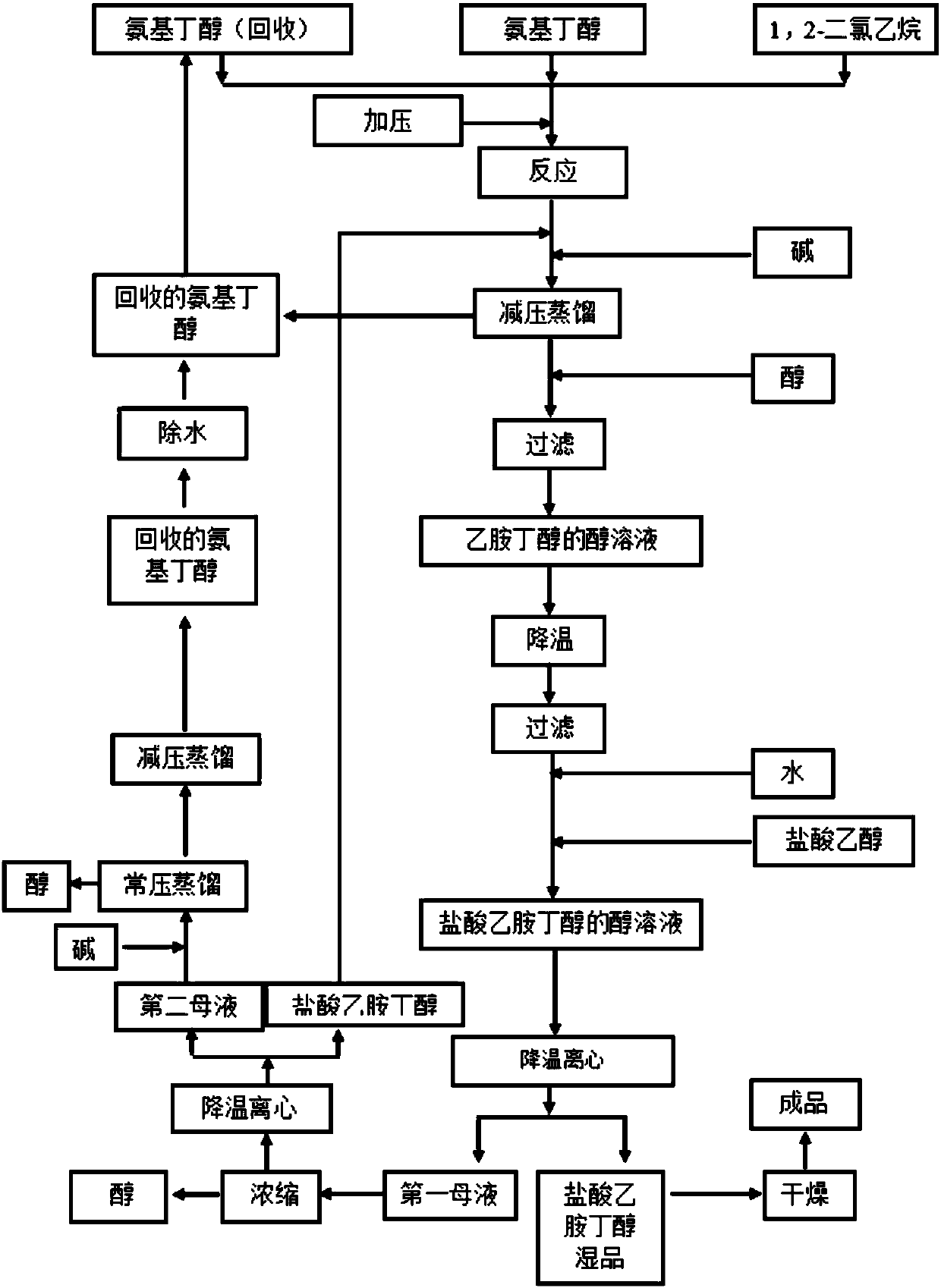
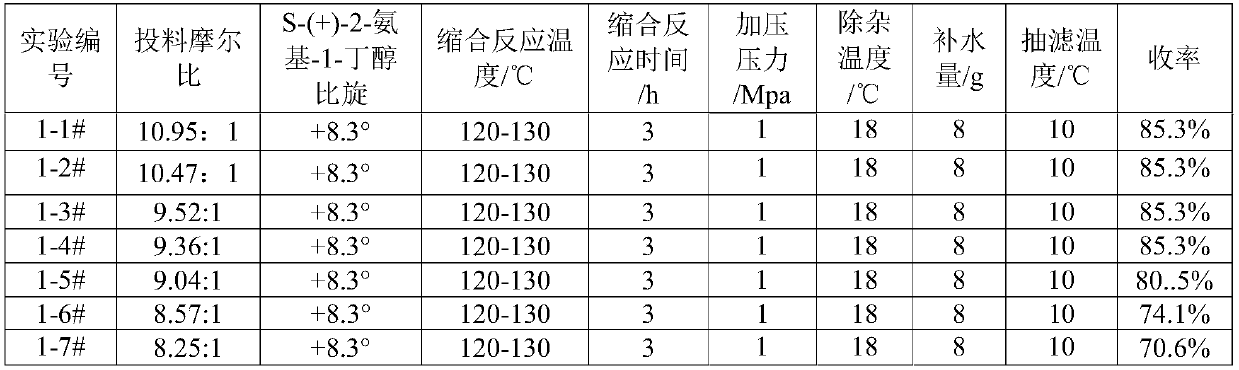
[0046] 295kg (3309.4mol) of S-(+)-2-amino-1-butanol (specific rotation +8.3°) was put into a 500L reactor, and under full stirring, 35kg (353.7mol) of 1,2 - Dichloroethane (the feeding ratio of aminobutanol to 1,2-dichloroethane is 9.36:1), pressurize to 1Mpa with nitrogen, stir and heat up to 110°C, control the temperature at 120°C-130°C to react 2 Hour. Cool down to 90°C and add 24.6kg (615mol) of sodium hydroxide in the air, keep it warm for 30 minutes, and recover 251.5kg (2821.4mol) of S-(+)-2-amino-1-butanol by vacuum distillation at 150°C. The vacuum pressure is -0.09MPa. Cool down to 70°C, add 200kg of absolute ethanol, stir for 30min, press filter into a 500L reactor, cool the filtrate to 15-20°C, press filter a 500L reactor, add 8kg of water, heat up to about 30°C, add 37.3kg of hydrochloric acid dropwise Ethanol (HCl content 30%), stirring, and controlling the pH between 3 and 3.5. Slowly lower the temperature to 8°C to 10°C, and centrifuge to obtain 83.6 kg of e...
Embodiment 2
[0051] With reference to the reaction conditions of Example 1, the condensation reaction temperature and the condensation reaction time were changed, and other conditions were unchanged to determine the impact on the product yield, see Table 2.
[0052] Table 2 Condensation reaction temperature and the impact table of reaction time on yield
[0053]
Embodiment 3
[0055] With reference to the reaction conditions of Example 1, the alcohol of the dissolved distillation product was changed alone, and other conditions were unchanged, to determine the impact on the product yield, see Table 3.
[0056] The influence table of the alcohol of table 3 dissolving distillation resultant on product yield
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com