Medical cermet material and preparation method thereof
A cermet and metal matrix technology, which is applied in the field of medical cermet materials and its preparation, can solve the problem of uneven distribution of metal and ceramic phases in wear-resistant composite materials, insufficient mixing of metal and ceramic particles, and wear-resistant composite materials. Poor abrasiveness and other problems to achieve the effect of low processing efficiency, good contact and high compressive strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
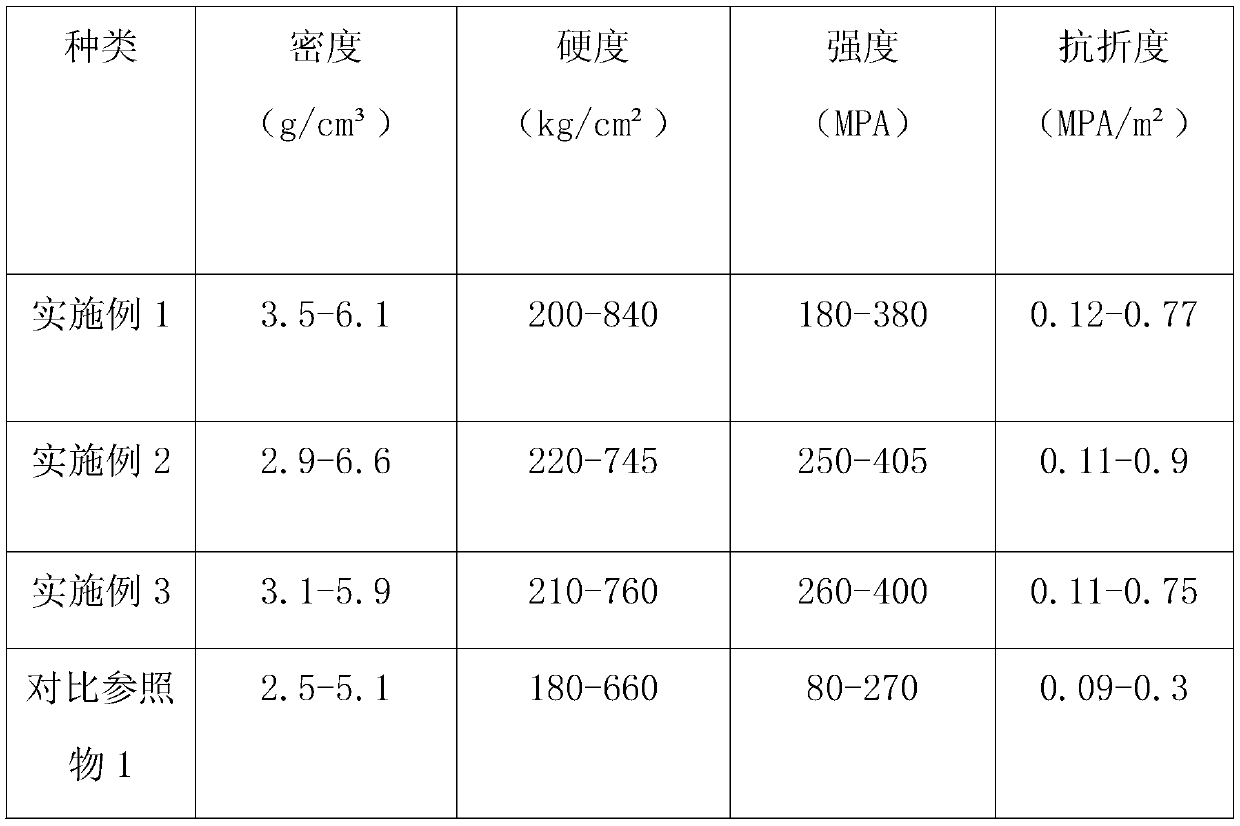
Embodiment 1
[0028] Embodiment 1, a medical cermet material and its preparation method, comprises the following components in parts by mass: 58 parts of metal matrix, 22 parts of ceramic particles, 4 parts of kaolin, and 1 part of binder.
[0029] Element metal substrate ceramic particles Kaolin Binding agent Ratio / part 58 22 4 1
[0030] A method for preparing a medical cermet material, comprising the following steps:
[0031] S1, Weigh the raw materials of each component according to the requirements and carry out mixing treatment;
[0032] S2, open the ball mill, carry out dry ball milling on the metal matrix, and ball mill to obtain small materials;
[0033] S3, after uniformly mixing the ceramic particles, the binder and the small material, trapping the material at 30°C for 16 hours, and mixing evenly after the trapping, to obtain the granular material;
[0034] S4, putting the granular material into the mold, and putting it into a drying furnace to obt...
Embodiment 2
[0045] Embodiment 2, a medical cermet material and its preparation method, comprises the following components in parts by mass: 60 parts of metal matrix, 24 parts of ceramic particles, 6 parts of kaolin, and 2 parts of binder.
[0046] Element metal substrate ceramic particles Kaolin Binding agent Ratio / part 60 24 6 2
[0047] S1, Weigh the raw materials of each component according to the requirements and carry out mixing treatment;
[0048] S2, open the ball mill, carry out dry ball milling on the metal matrix, and ball mill to obtain small materials;
[0049] S3, after uniformly mixing the ceramic particles, the binder and the small material, trapping the material at 38°C for 28 hours, and mixing evenly after the trapping, to obtain the granular material;
[0050] S4, putting the granular material into the mold, and putting it into a drying furnace to obtain a dry billet;
[0051] S5, put the dry billet in step S3 into a hot-press furnace, rai...
Embodiment 3
[0061] Embodiment 3, a medical cermet material and its preparation method, comprising the following components in parts by mass: 63 parts of a metal matrix, 26 parts of ceramic particles, 8 parts of kaolin, and 3 parts of a binder.
[0062] Element metal substrate ceramic particles Kaolin Binding agent Ratio / part 63 26 8 3
[0063] A method for preparing a medical cermet material, comprising the following steps:
[0064] S1, Weigh the raw materials of each component according to the requirements and carry out mixing treatment;
[0065] S2, open the ball mill, carry out dry ball milling on the metal matrix, and ball mill to obtain small materials;
[0066] S3, after uniformly mixing the ceramic particles, the binder and the small material, trapping the material at 42°C for 48 hours, and mixing evenly after the trapping, to obtain the granular material;
[0067] S4, putting the granular material into the mold, and putting it into a drying furnace ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com