Transdermal absorption preparation for treating asthma
A technology for transdermal absorption preparations and plasticizers, which is applied in the field of pharmaceutical preparations, can solve problems such as the increase in the impurity content of active ingredients, and achieve the effects of reducing the production of pharmaceutical magazines, enhancing plasticity, and improving plasticity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
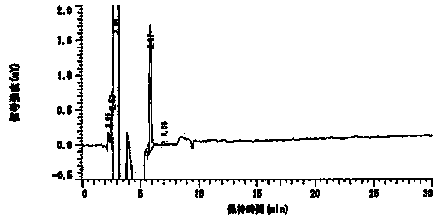
Embodiment 1
[0044] The composition of the prescription is as follows:
[0045] Preparation components Content (parts) DURO-TAK 87-2287 60.2 Toloterol 4.8 Diethanolamine Lauric Acid 3.0 EUDRAGIT ® RE 100
[0046] Preparation method: weigh EUDRAGIT in the prescribed amount ® RE 100 is placed in a container, and the prescribed amount of ethyl acetate is added to stir the solution to obtain EUDRAGIT ® RE 100 solution; weigh the prescribed amount of acrylate pressure-sensitive adhesive and hydrogenated rosin glyceride into a container, add the freshly prepared EUDRAGIT ® RE 100 solution was stirred to obtain a binder solution; the prescribed amount of tulobuterol was added to the prescribed amount of ethanol to dissolve, and then the prescribed amount of diethanolamine lauric acid and diisopropanolamine were added to continue stirring and dissolving. Obtain tulobuterol ethanol solution; mix tulobuterol ethanol solution with adhesive solution to obtain a co...
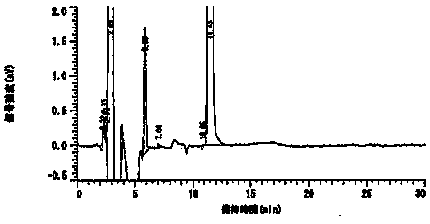
Embodiment 2
[0048] The composition of the prescription is as follows:
[0049] Preparation components Content (parts) DURO-TAK 87-2287 55.2 Toloterol 4.8 Diethanolamine Lauric Acid 3.0 EUDRAGIT ® RE
[0050] Preparation method: weigh EUDRAGIT in the prescribed amount ® RE is placed in a container, and the ethyl acetate of the prescription amount is added to stir the solution to obtain EUDRAGIT ® RE solution; weigh the prescribed amount of acrylate pressure-sensitive adhesive and rosin glyceride into the container, add the freshly prepared EUDRAGIT ® RE solution is stirred to obtain a binder solution; the tulobuterol of the prescription quantity is added to the ethanol of the prescription quantity for dissolving, and then the diethanolamine lauric acid and diisopropanolamine of the prescription quantity are added to continue stirring and dissolving to obtain Tulobuterol ethanol solution; mix the tulobuterol ethanol solution with the adhesive solution t...
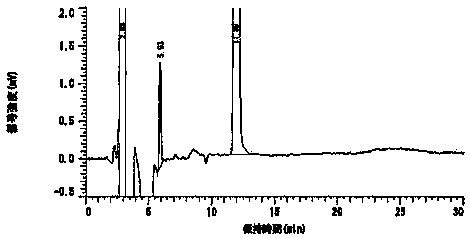
Embodiment 3
[0052] The composition of the prescription is as follows:
[0053] Preparation components Content (parts) DURO-TAK 87-2510 54.2 Toloterol 4.8 Diethanolamine Lauric Acid 3.0 EUDRAGIT ® RE
[0054] Preparation method: weigh EUDRAGIT in the prescribed amount ® RE100 is placed in a container, and the prescribed amount of ethyl acetate is added to stir the solution to obtain EUDRAGIT ® RE solution; weigh the prescribed amount of acrylate pressure-sensitive adhesive and rosin glyceride into the container, add the freshly prepared EUDRAGIT ® RE 100 solution was stirred to obtain a binder solution; the prescribed amount of tulobuterol was added to the prescribed amount of ethanol to dissolve, and then the prescribed amount of diethanolamine lauric acid and diisopropanolamine were added to continue stirring and dissolving. Obtain tulobuterol ethanol solution; mix tulobuterol ethanol solution with adhesive solution to obtain a colloid mixed solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com