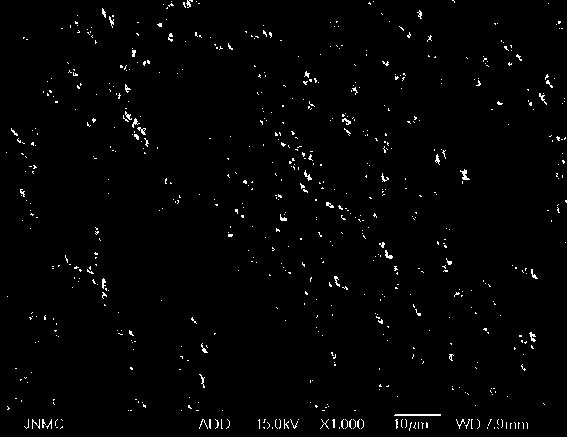
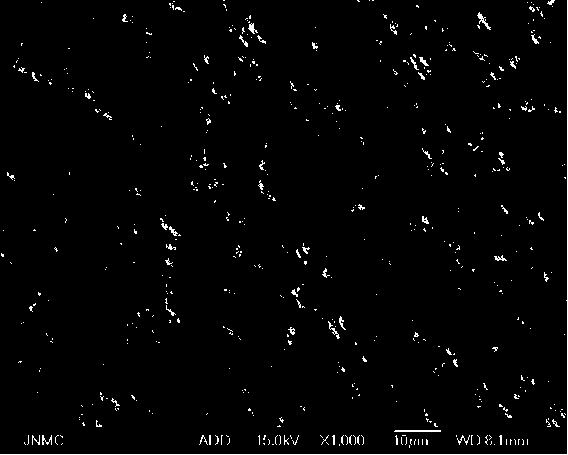
Preparation method of metal coated cobaltosic oxide
A technology of tricobalt tetroxide and cobalt hydroxide, which is applied in the field of lithium-ion batteries, can solve the problems of easy hydrolysis, low production efficiency, uneven cobalt precipitation, etc., and achieve the effect of inhibiting growth and increasing the number of crystal nuclei
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The production steps are the same as above, and will not be repeated here. The specific parameters in each step are as follows:
[0057] The configured A solution is 10m 3 The cobalt concentration is 20g / L cobalt nitrate solution; the prepared B solution is 10m 3 Cobalt nitrate solution with a cobalt concentration of 160g / L; the prepared C solution is a mixed solution of sodium hydroxide solution and ammonia solution. Among them, the concentration of sodium hydroxide solution is 200g / L, the concentration of ammonia solution is 180g / L, 10ml of hydrazine hydrate solution with a mass concentration of 80% is added to every liter of ammonia solution, and the volume of ammonia solution and sodium hydroxide solution in C solution The ratio is 0.05; the configured solution D is 500L aluminum nitrate solution with an aluminum concentration of 9.86g / L.
[0058] Synthesis reaction: At the beginning of the synthesis reaction, add solution A to solution B at a flow rate of 250L / h...
Embodiment 2
[0066] The production steps are the same as above, and will not be repeated here. The specific parameters in each step are as follows:
[0067] The configured A solution is 10m 3 The cobalt concentration is 30g / L cobalt sulfate solution, and the prepared B solution is 10m 3 The cobalt sulfate solution that cobalt concentration is 150g / L. The prepared C solution is a mixed solution of sodium hydroxide solution and ammonia solution, wherein the concentration of sodium hydroxide solution is 250g / L, the concentration of ammonia solution is 180g / L, and 15ml of hydration solution with a mass concentration of 80% is added to each liter of ammonia solution. Hydrazine solution, and the volume ratio of ammonia solution to sodium hydroxide solution in solution C is 0.08; solution D is 500L aluminum sulfate solution with an aluminum concentration of 14.79g / L.
[0068] Synthesis reaction: At the beginning of the synthesis reaction, add solution A to solution B at a flow rate of 250L / h, ...
Embodiment 3
[0076] The production steps are the same as above, and will not be repeated here. The specific parameters in each step are as follows:
[0077] The configured A solution is 10m 3 The cobalt concentration is 40g / L cobalt chloride solution; the prepared B solution is 10m 3 A cobalt chloride solution with a cobalt concentration of 140g / L; the prepared solution C is a mixed solution of sodium hydroxide solution and ammonia solution. Among them, the concentration of sodium hydroxide solution is 300g / L, the concentration of ammonia solution is 180g / L, 20ml of hydrazine hydrate solution with a mass concentration of 80% is added to every liter of ammonia solution, and the volume of ammonia solution and sodium hydroxide solution in C solution The ratio is 0.1; the configured D solution is 500L aluminum trichloride solution with an aluminum concentration of 119.73g / L.
[0078] Synthesis reaction: At the beginning of the synthesis reaction, add solution A to solution B at a flow rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com