Vaccine composition and preparation method thereof
A vaccine composition and the technology of the composition are applied in the direction of biochemical equipment and methods, vaccines, and veterinary vaccines, which can solve problems such as adverse effects, antigen interference effects, and loss of efficacy, and achieve small side effects, simplified immunization procedures, and vaccination. The effect of fewer seedlings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation and testing of PCV2-PRV-PPV-PRRSV quadruple inactivated vaccine composition
[0044] 1. Collect antigens
[0045] 1.1 Preparation of poisonous seeds for production
[0046] 1.1.1 Preparation of PCV2 SH strain:
[0047] Properly dilute the virus seed of PCV2 SH strain with virus diluent (i.e., serum-free MEM cell maintenance solution), inoculate it in a monolayer PK-15 cell culture at 0.01 MOI (multiplicity of infection), absorb at 37°C for 30 minutes, add 4% (v / v) Calf serum and 2mmol / L D-glucosamine hydrochloride MEM cell maintenance solution, cultured at 37°C for 4 days, frozen 2-3 times, harvested SH strain virus liquid, stored below -20°C, should be Not more than 2 months.
[0048] 1.1.2 Preparation of Bartha-K61 strain:
[0049] Properly dilute the Bartha-K61 strain virus seed with the virus diluent (i.e., serum-free MEM cell maintenance solution), inoculate it in a monolayer PK-15 cell culture at 0.01 MOI (multiplicity of infection), absorb at 37°C f...
Embodiment 2- Embodiment 5 and comparative example 1
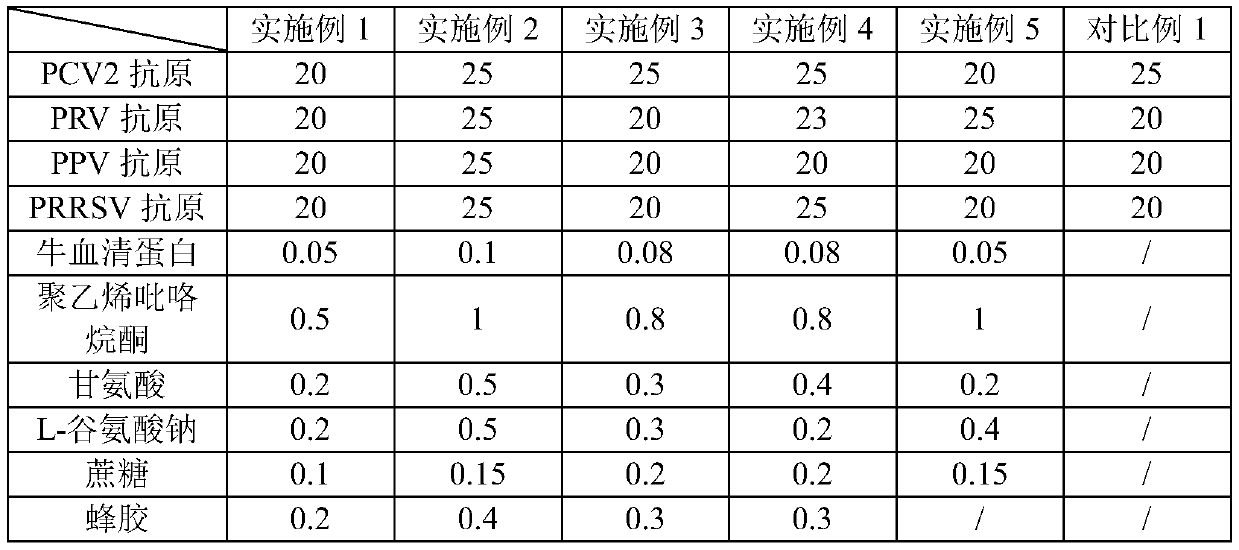
[0097] Embodiment 2-Example 5 and Comparative Example 1 are all based on the method of Example 1, and the components and contents of the vaccine composition are adjusted. The specific adjustments and the components and contents of Example 1 are shown in Table 2 below.
[0098] The components and content of the vaccine composition of table two embodiment 1-embodiment 5 and comparative example 1
[0099]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com