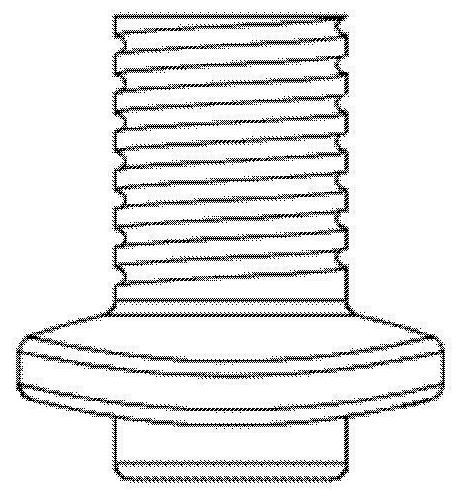
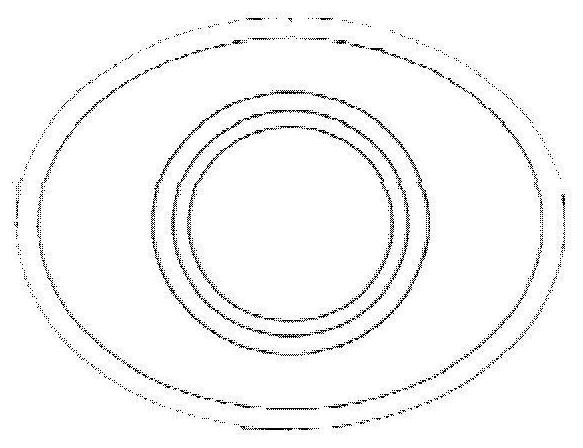
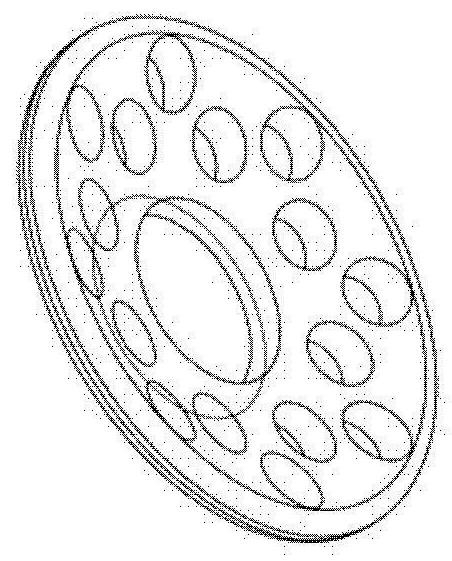
Artificial corneas containing biomaterials
A technology of artificial cornea and biomaterials, applied in the field of medicine, to reduce the chance of entering the eye, reduce foreign body reactions, and facilitate large-scale promotion and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0067] The present invention uses the exposure rate of the artificial corneal stent to evaluate the implementation effect of the artificial cornea of the present invention, and the exposure rate is also the rejection rate. According to the applicant's experience of using MICOF and Boston type II artificial corneas, the exposure rate (rejection rate) of patients implanted with existing PMMA artificial corneas without biomaterial components is 60% 5 years after the operation. However, the applicant performed a total of 156 cases of artificial cornea implantation operations containing autologous ear cartilage. As a result, only 6 cases were exposed after 5 years, and the exposure rate dropped to 3.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com