A kind of synthetic method of linear epoxy resin containing multiple glycidyl ether groups
A glycidyl ether-based, linear epoxy resin technology, applied in the field of online epoxy resin synthesis, can solve difficult problems and achieve excellent alkali resistance and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
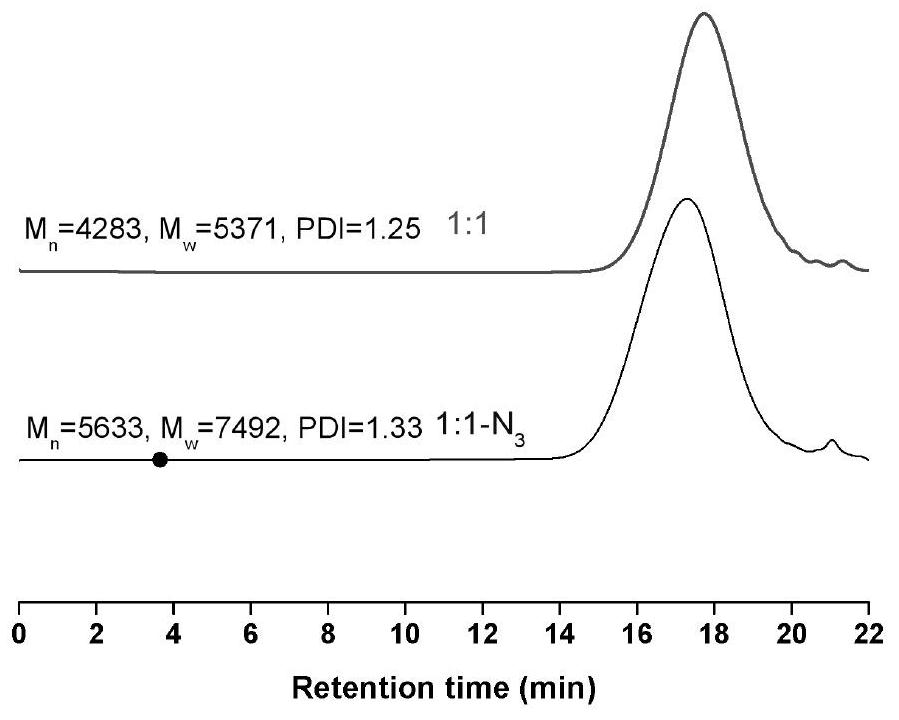
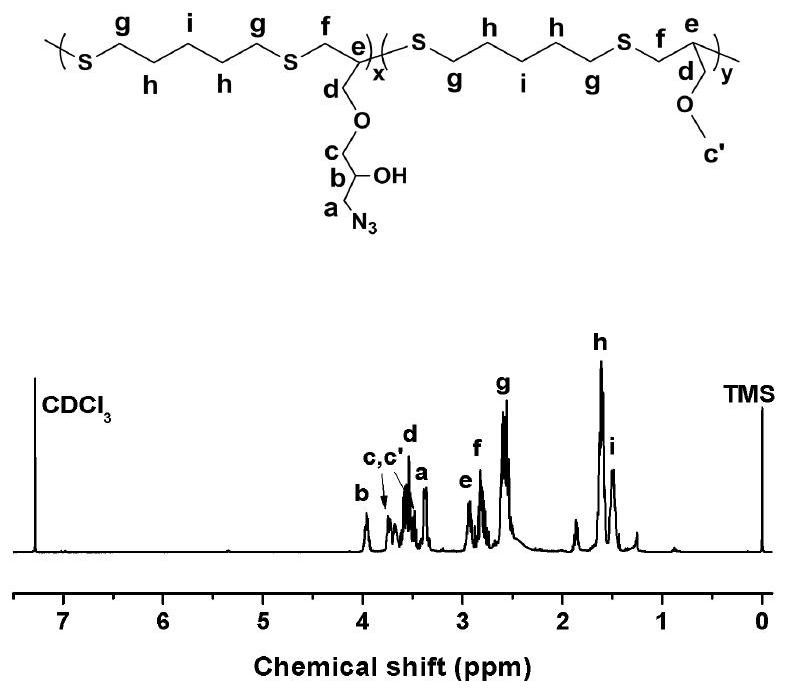
[0044] Embodiment 1-4 is the specific implementation example of reaction after mixing propargyl glycidyl ether and propargyl methyl ether in different molar ratios, wherein the molar ratio of the two in embodiment 1 is 1:1, and in embodiment 2, two The molar ratio of the two is 2:1, the molar ratio of the two is 1:2 in embodiment 3, and the molar ratio of the two is 1:4 in embodiment 4.
[0045] Example 1
[0046] A method for synthesizing a linear epoxy resin containing multiple glycidyl ether groups, comprising the following steps;
[0047] S1: Mix the raw material propargyl glycidyl ether and propargyl methyl ether according to the molar ratio of 1:1, and then react with the raw material 1,5-pentanedithiol according to the molar ratio of alkynyl and mercapto groups of 1:2 In the container, add the benzoin dimethyl ether of 5% of the total mass of the raw materials and the 1,4-dioxane of 200% of the total mass of the raw materials, in an ice-water bath, ultraviolet light ir...
Embodiment 2
[0058] A synthetic method of a linear epoxy resin containing multiple glycidyl ether groups, the synthetic method is the same as in Example 1, the difference is that in step S1 the raw material propargyl glycidyl ether and propargyl methyl ether are in a molar ratio of 2 :1 mix.
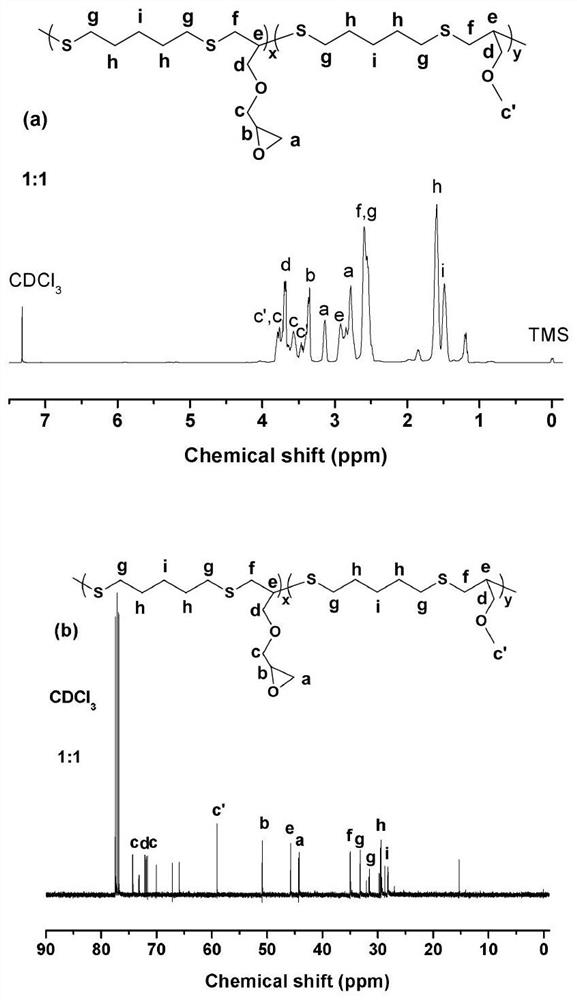
[0059] The structure of the product (linear epoxy resin containing multiple glycidyl ether groups) obtained in embodiment 2 has been characterized with nuclear magnetic resonance spectrum, see Figure 6 . The chemical shifts of hydrogen protons in different chemical environments in the product of Example 2 have been assigned, and have been marked in the spectrogram, such as Figure 6 Shown in (a), the absorption peak at chemical shift 2.5-3.2ppm is the chemical shift of hydrogen on the epoxy group, as Figure 6 As shown in (b), the corresponding chemical shifts in the carbon nuclear magnetic spectrum appear at 44.2ppm and 50.9ppm respectively, indicating that the epoxy group has successfully entere...
Embodiment 3
[0066] A kind of synthetic method of the linear epoxy resin that contains a plurality of glycidyl ether groups, synthetic method is the same as embodiment 1, difference is that in step S1 raw material propargyl glycidyl ether and propargyl methyl ether according to molar ratio 1 :2 mixed.
[0067] The structure of the product (linear epoxy resin containing multiple glycidyl ether groups) obtained in embodiment 3 has been characterized by nuclear magnetic resonance spectrum ( Figure 8 ), indicating that the epoxy group has successfully entered the structure of the copolymerization product and proves that the mercapto-alkyne copolymerization reaction adopted in this example is successful.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com