Preparation method of thienopyridine-type ionic rod-like liquid crystal and ionic liquid compound
An ionic liquid and compound technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problem of high price of pyridine and achieve the effect of rich chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of raw material thieno[3,2-c]pyridine
[0050] A preparation method of thieno[3,2-c]pyridine heterocyclic compound 3, comprising the following steps: (1) using 4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride Neutralization reaction occurs under alkaline conditions to obtain compound 2; (2) compound 2 and manganese dioxide are stirred and refluxed in toluene solvent to undergo aromatization reaction to obtain compound 3; the synthetic route is as follows:
[0051]
[0052] Add 4,5,6,7-tetrahydrothiophene[3,2-c]pyridine hydrochloride (1.0 g, 5.69 mmol) and sodium hydroxide (228 mg, 5.69 mmol) to water (20 mL) and dichloromethane (20mL), stirred and reacted at room temperature for 1-2 hours, extracted with dichloromethane, dried over magnesium sulfate and filtered, and distilled off the solvent (be careful not to depressurize, the product has sublimation properties); Manganese dioxide (3.5g, 39.8mmol) was activated at 230°C for 2-3 hou...
Embodiment 2
[0054] Example 2: Ionization of bromoperfluorohexylpropane, when X is Br -
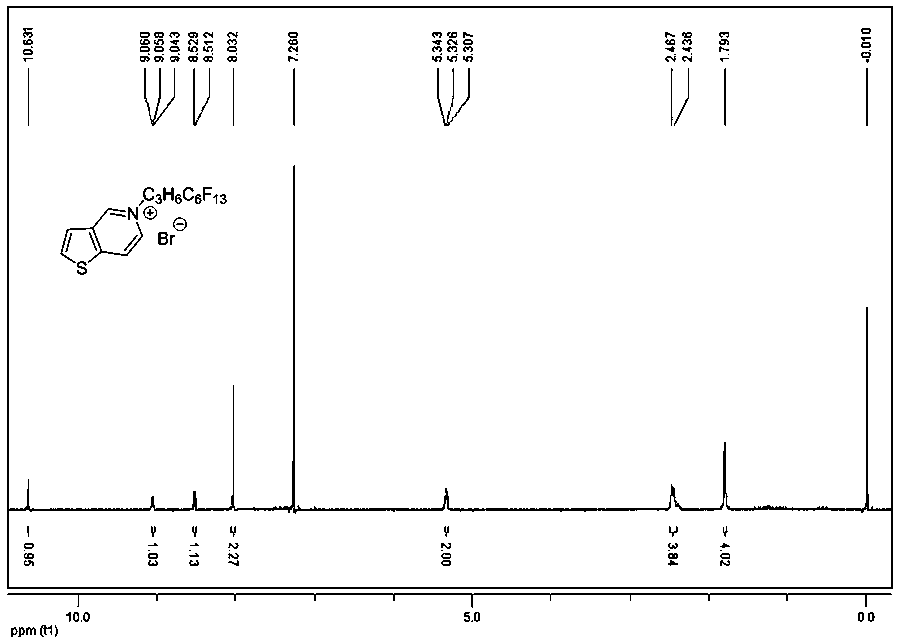
[0055] A preparation method of a thienopyridine-type ionic rod-shaped liquid crystal compound, comprising the following steps: using thieno[3,2-c]pyridine as a raw material, and bromoperfluorohexylpropane (Br-C 3 h 6 C 6 f 13 ) heated and stirred to reflux, and reacted to obtain compound 4; its synthetic route is as follows:
[0056]
[0057] Thieno[3,2-c]pyridine (100mg, 0.74mmol) and Br-C 3 h 6 C 6 f 13 Add (978.77mg, 2.22mmol) into a small round bottom flask with a capacity of 10mL, add 2-3mL toluene as a solvent, heat and stir at 120°C, condense and reflux for about 12 hours, and use thin-layer chromatography to determine that the reaction of the raw materials is complete. Stop the reaction. After the reaction, it was purified by silica gel column chromatography (dichloromethane:ethanol=10:1) to obtain compound 4 (332 mg), with a yield of 77.89%; macroscopically, it was not easy to bec...
Embodiment 3
[0060] Embodiment 3: ion exchange, this moment X is I -
[0061] A preparation method of a thienopyridine-type ionic rod-shaped liquid crystal compound, comprising the following steps: using thieno[3,2-c]pyridine as a raw material, and bromoperfluorohexylpropane (Br-C 3 h 6 C 6 f 13 ) after ionization, use KI to perform ion exchange, and react to obtain compound 5; its synthetic route is as follows:
[0062]
[0063] Add compound 4 (100mg, 0.17mmol) and KI (144.05mg, 0.87mmol) into a small round bottom flask with a capacity of 10mL, add 2-3mL dichloromethane and 1-2mL water as solvents, and stir the reaction at room temperature After about 1-2 hours, the reaction is terminated after confirming that the raw materials are completely reacted by thin-layer chromatography. After the reaction, extract with dichloromethane (note that it cannot be chemically dried to prevent the introduction of impurity ions), then distill under reduced pressure on a rotary evaporator, and obs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com