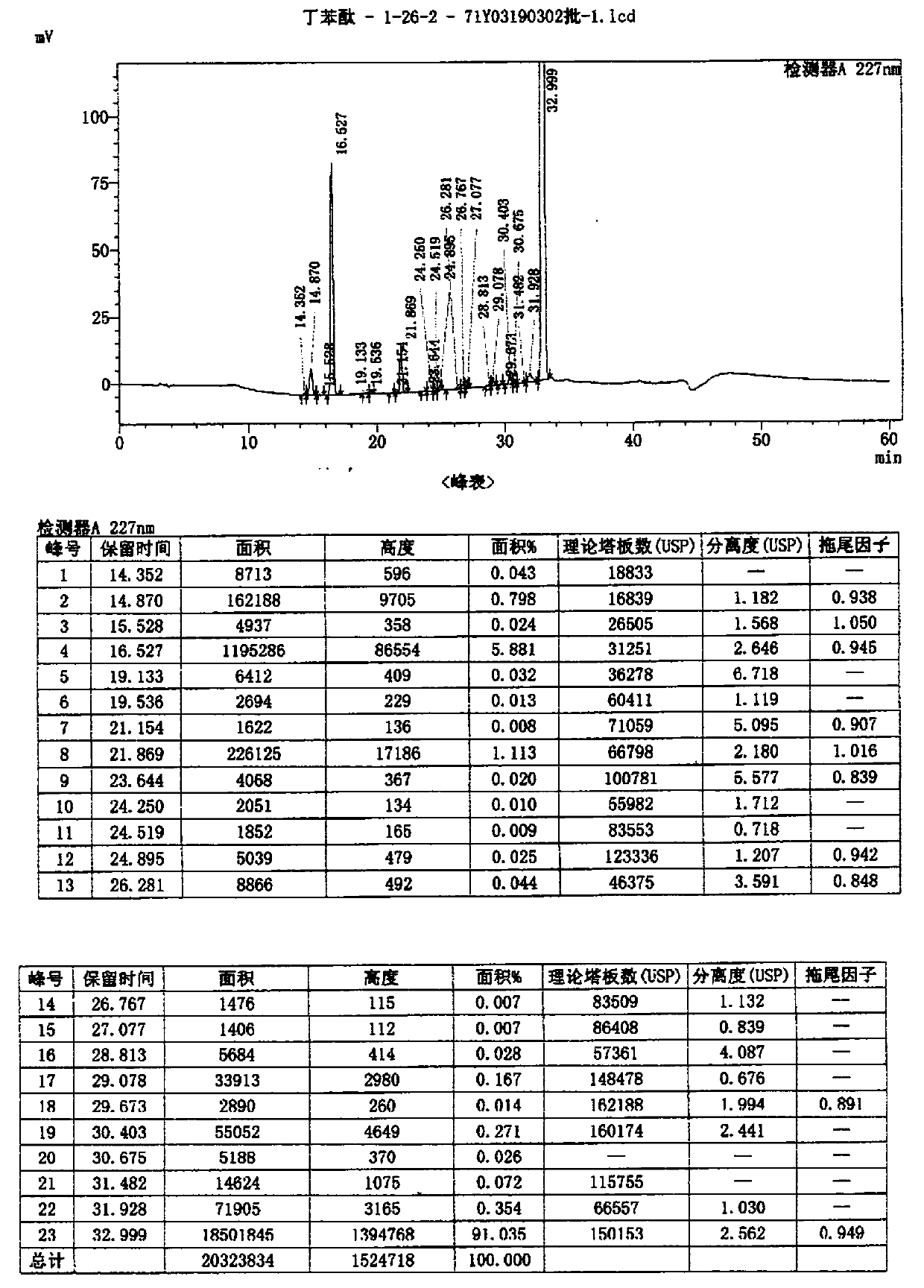
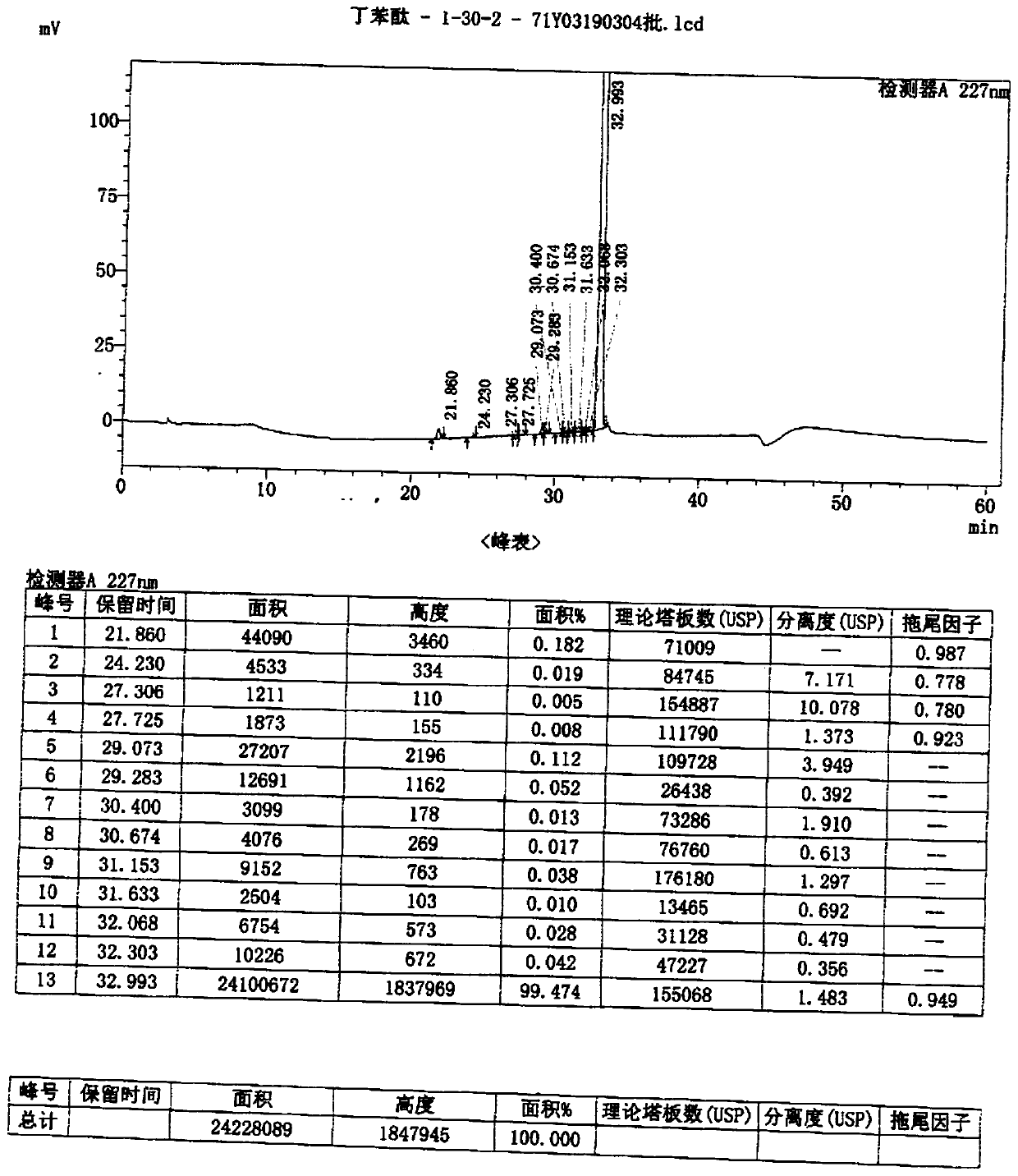
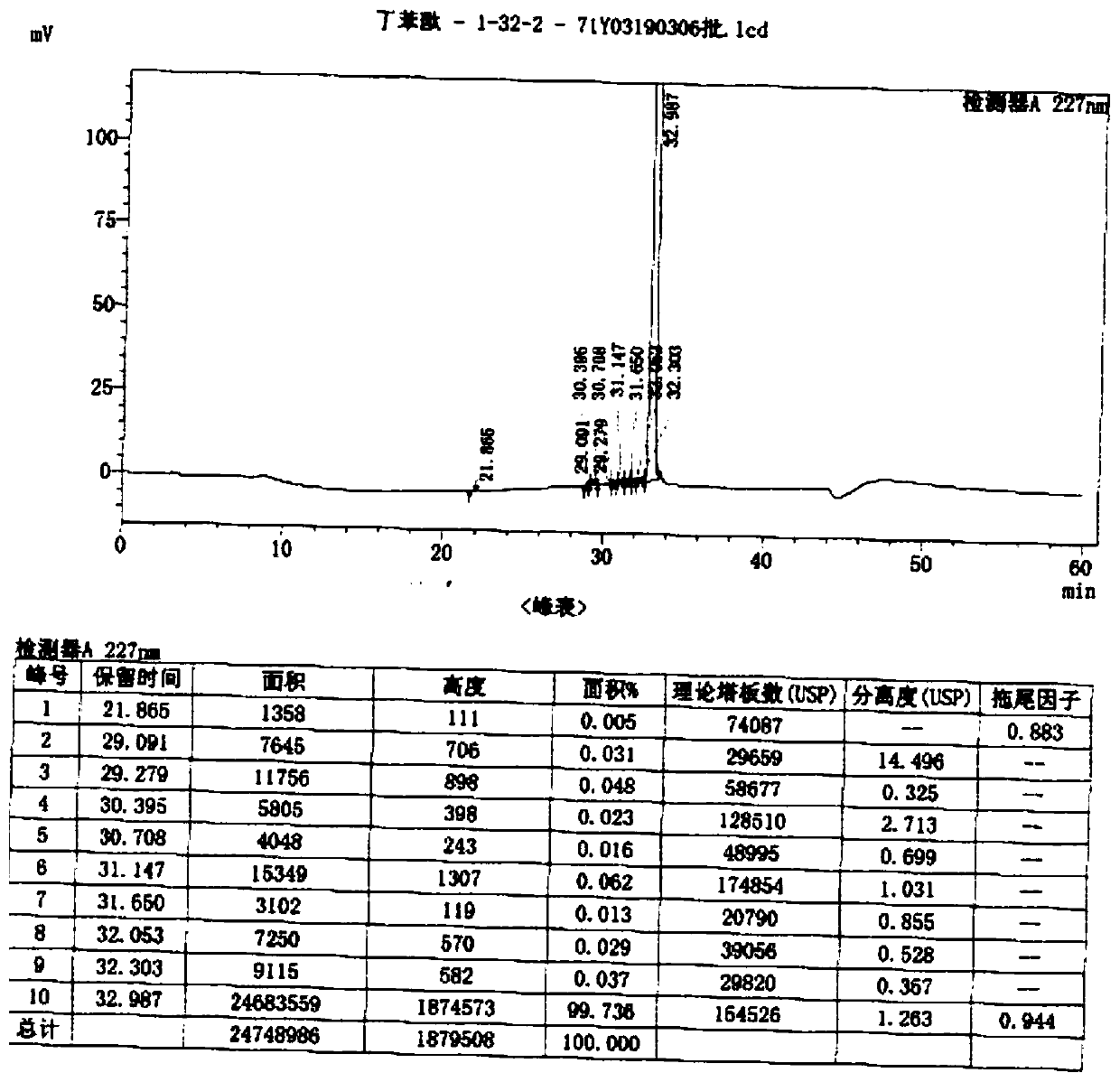
Method for preparing high-purity butyphthalide
A butylphthalide, high-purity technology, applied in the field of drug synthesis, can solve the problems of poor stability of format reagents, long reaction routes, cumbersome operations, etc., and achieve the effects of easy industrial production, stable batch supply, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The microchannel reactor was cooled to -30°C for pre-cooling, 100 g (0.666 mol) of o-formylbenzoic acid was dissolved in 600 mL of tetrahydrofuran, and 606 mL of n-butyllithium in n-hexane (2.2 mol / L). Put the THF solution of o-formylbenzoic acid into the first piece of the microchannel; inject the n-butyllithium n-hexane solution into the second piece of the microchannel, and mix in the third piece of the microchannel, with a total flow rate of 30mL / min. After the reaction was complete, 3N hydrochloric acid aqueous solution was added, stirred for 30 minutes, allowed to stand for liquid separation, and the lower aqueous phase (pH=2) was extracted twice with 300 mL of dichloromethane. The organic phases were combined, dried and concentrated to obtain 126 g of a yellow oil.
[0054] Take 143g of crude product, add methanol 715mL, sodium hydroxide 26.6g, heat to reflux for 1hrs, and the reaction is complete; concentrate to remove methanol, add 430mL of dichloromethane, wate...
Embodiment 2
[0057] The microchannel reactor was cooled to -30°C for pre-cooling, 100 g (0.666 mol) of o-formylbenzoic acid was dissolved in 600 mL of tetrahydrofuran, and 606 mL of n-butyllithium in n-hexane (2.2 mol / L). Put the THF solution of o-formylbenzoic acid into the first piece of the microchannel; inject the n-butyllithium n-hexane solution into the second piece of the microchannel, and mix in the third piece of the microchannel, with a total flow rate of 50mL / min. Add 10% sodium hydroxide aqueous solution to the microchannel effluent, stir for 30 minutes, let stand to separate the liquid, discard the organic phase, adjust the pH to 2 with 55 mL of concentrated hydrochloric acid in the aqueous phase, precipitate solid, stir for 30 minutes, and suction filter to obtain shallow yellow solid.
[0058] Add the solid to the reaction flask, add 300 mL of dichloromethane, 100 mL of water, and 50 mL of concentrated hydrochloric acid, and heat to reflux for 1 hrs. The feed liquid is cool...
Embodiment 3
[0061] The microchannel reactor was cooled to -30°C for pre-cooling, 100 g (0.666 mol) of o-formylbenzoic acid was dissolved in 600 mL of tetrahydrofuran, and 606 mL of n-butyllithium in n-hexane (2.2 mol / L). Put the THF solution of o-formylbenzoic acid into the first piece of the microchannel; 300mL of n-butyllithium n-hexane solution into the second piece of the microchannel, after mixing in the third piece of the microchannel reactor, in the fourth Pour the remaining 306 mL of tetrahydrofuran solution of n-butyllithium into the main pipeline of the microchannel with a total flow rate of 100 mL / min. Add 10% sodium hydroxide aqueous solution to the microchannel effluent, stir for 30 minutes, let stand to separate the liquid, discard the organic phase, adjust the pH to 2 with 55 mL of concentrated hydrochloric acid in the aqueous phase, precipitate solid, stir for 30 minutes, and suction filter to obtain shallow yellow solid.
[0062] Add the solid to the reaction flask, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com