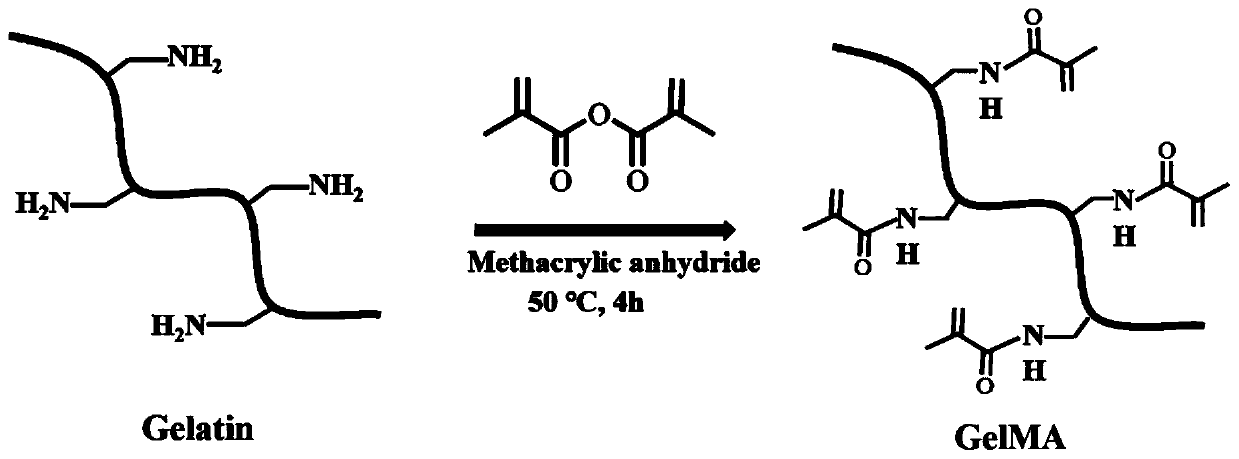
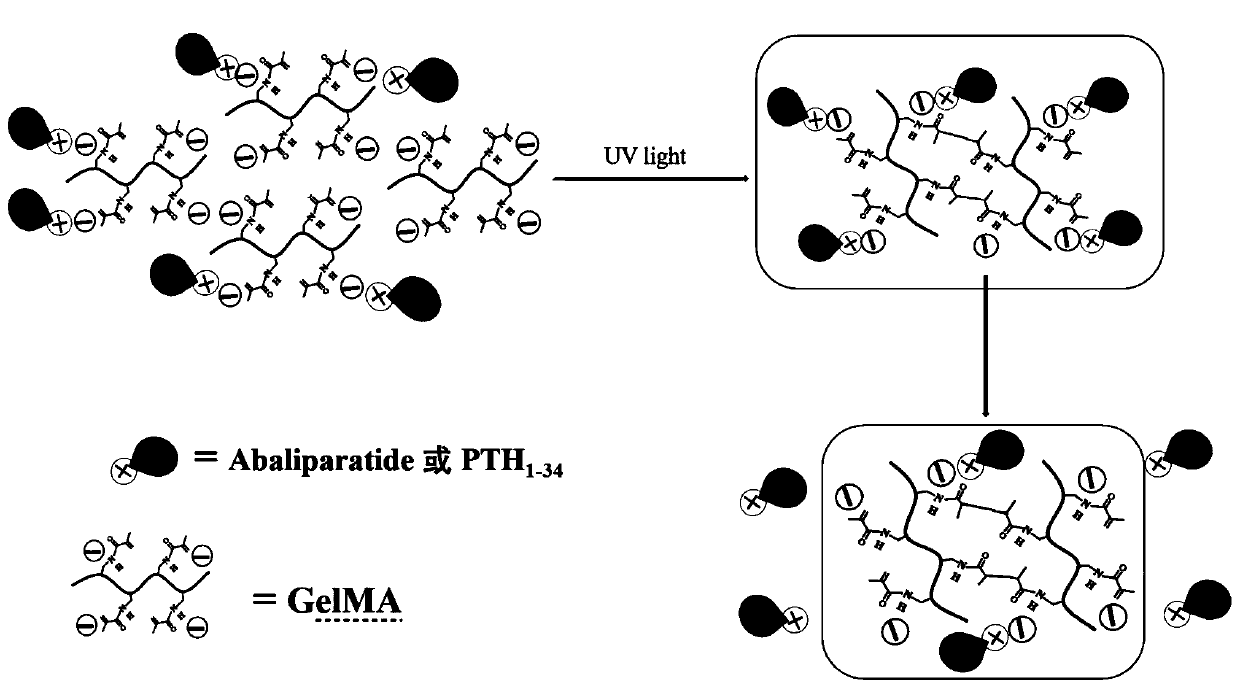
Preparation method and application of GelMA hydrogel for locally slow-releasing Abaloparatide or related polypeptides
A hydrogel, topical technology, used in medical science, prosthesis, tissue regeneration, etc., to achieve good biocompatibility and promote biological behavior.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the GelMA hydrogel capable of local sustained release of Abaloparatide or related polypeptide analogs of the present invention comprises the following steps:
[0034] (1) Weigh methacrylic anhydride gelatin (GelMA) monomer and dissolve it in PBS solution, the mass fraction of GelMA is 5-30%, shake and dissolve at 37-50°C and avoid light for 15-20min;
[0035] (2) Take the LAP blue photoinitiator and mix it with the GelMA solution prepared in step (1) at room temperature and avoid light, and the mass fraction of the LAP photoinitiator in the solution is 0.1% to 1%. Use a sterile filter to dissolve the solution Filter into a sterile centrifuge tube;
[0036] (3) Abaloparatide or PTH 1-34 Dissolve in PBS solution, then add dropwise to the solution in step (2), mix ultrasonically for 20-60 minutes at room temperature, let stand for 3-24 hours, and then use a blue light source (excitation wavelength 400-490nm) to irradiate the mixed solution for 3-...
Embodiment 1
[0041] Preparation of GelMA hydrogel for local sustained release of Abaloparatide:
[0042] (1) Weigh 0.25g of GelMA monomer and dissolve it in 5ml of PBS solution, shake and dissolve at 37°C for 15min (under dark conditions), and prepare a GelMA solution with a mass fraction of 5%;
[0043] (2) Weigh 0.005g of LAP blue photoinitiator and add it to the solution of (1) above and mix evenly (under dark conditions), then filter the solution into a new sterile centrifuge tube with a 0.22 μm filter membrane.
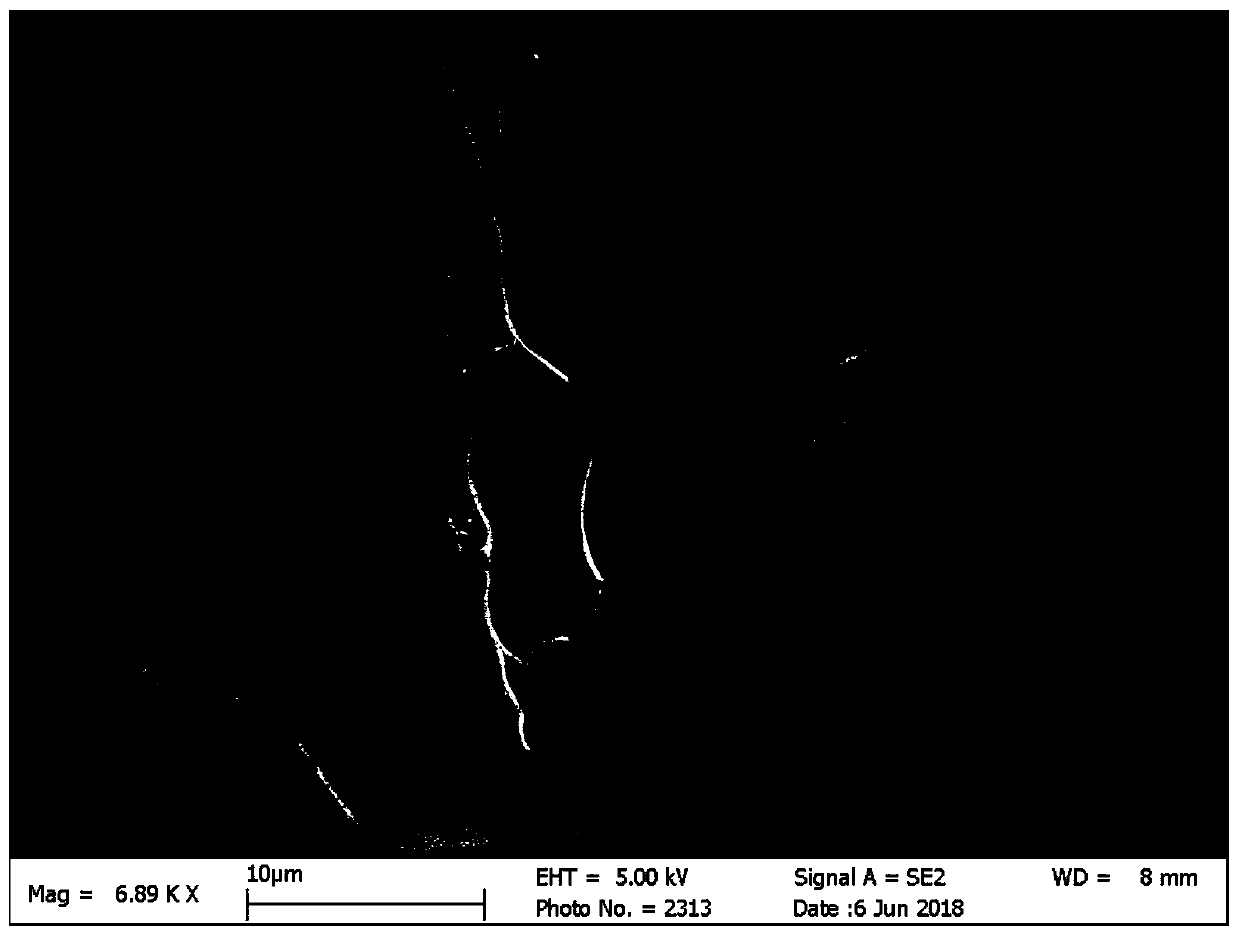
[0044] (3) Dissolve Abaloparatide in PBS solution, then add it dropwise to the above GelMA solution and mix it ultrasonically for 20 minutes. The final concentration of Abaloparatide in the solution is 0.5mg / mL. After standing for 3 hours, use a blue light source (excitation wavelength 405nm) to irradiate Mix the solution and solidify into a gel after 3s. By scanning electron microscope (SEM) test ( image 3 ) It can be seen that Abaloapratide is attached to the hydrogel gr...
Embodiment 2
[0046] Preparation of GelMA hydrogel for local sustained release of Abaloparatide:
[0047] (1) Weigh 0.25g of GelMA monomer and dissolve it in 5ml of PBS solution, shake and dissolve at 37°C for 15min (under dark conditions), and prepare a GelMA solution with a mass fraction of 5%;
[0048] (2) Weigh 0.005g of LAP blue photoinitiator and add it to the solution of (1) above and mix evenly (under dark conditions), then filter the solution into a new sterile centrifuge tube with a 0.22 μm filter membrane.
[0049] (3) Dissolve Abaloparatide in PBS solution, then add it dropwise to the above GelMA solution and mix it ultrasonically for 40 minutes. The final concentration of Abaloparatide in the solution is 0.5 mg / mL. After standing for 10 hours, use blue light source (excitation wavelength 405nm) to irradiate Mix the solution and let it solidify into a gel for 5s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com