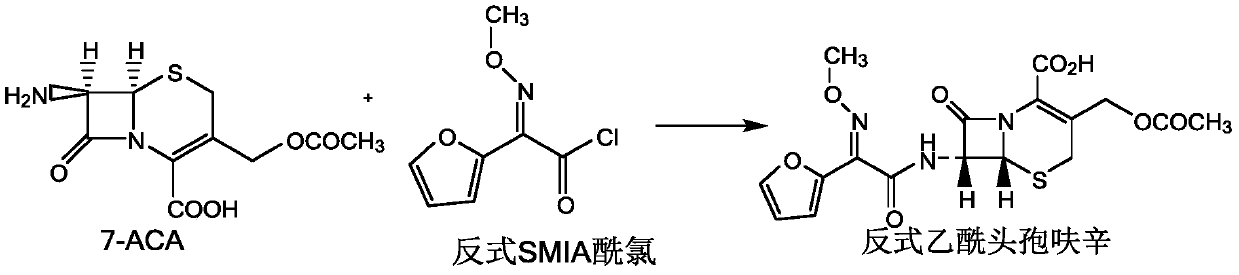
Preparation method of anti-form cefuroxime derivative
A technology for trans-cefturoxime derivatives, which is applied in the field of preparation of trans-cefturoxime derivatives, can solve the problems of high preparation cost and low yield, and achieve the effect of efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The present invention relates to a kind of preparation method of trans cefuroxime derivatives, comprising:
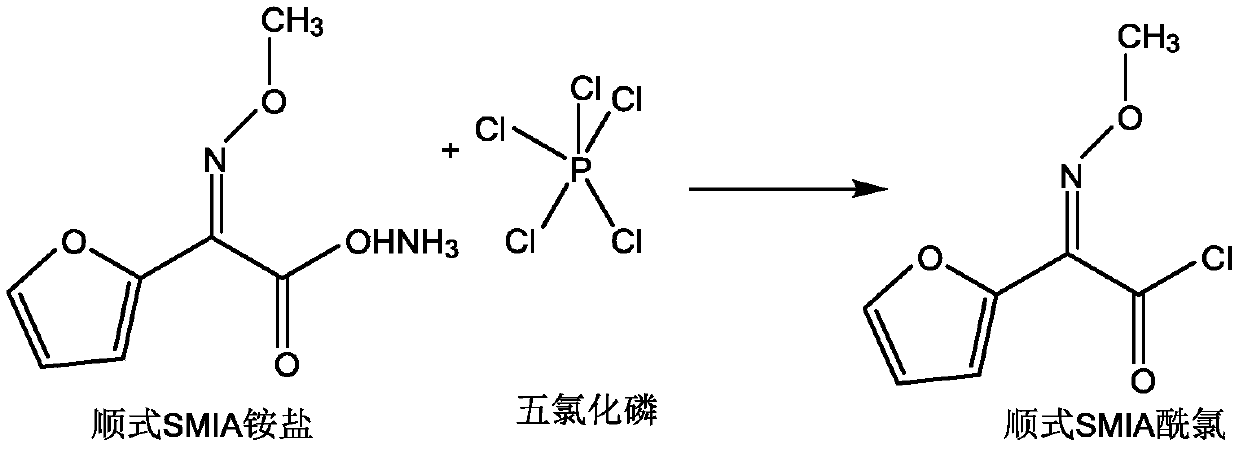
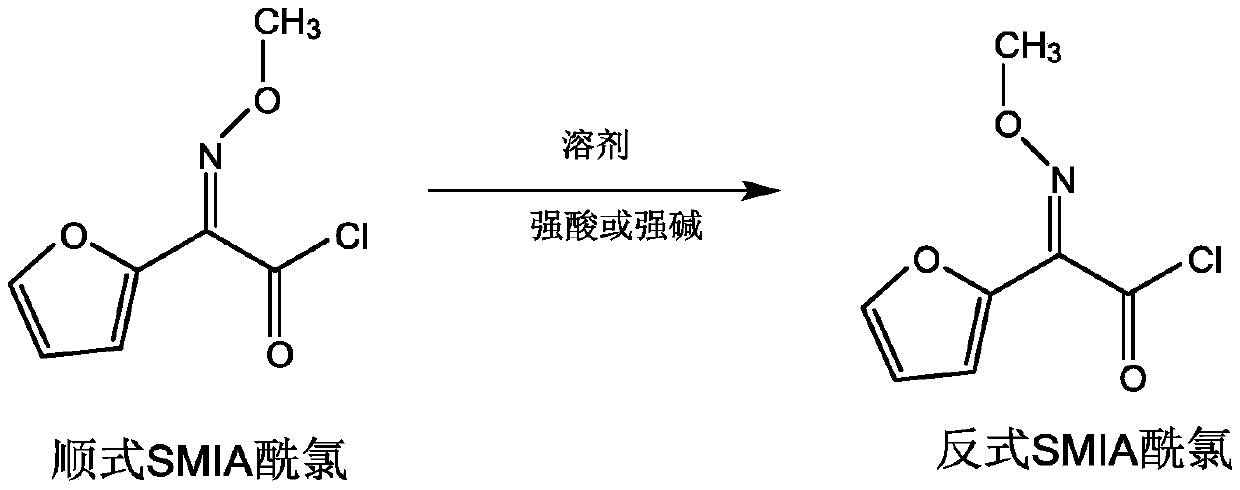
[0020] A. Dissolve phosphorus pentachloride in a solvent, lower the temperature to -30-10°C, and add cis-methoxyiminofuran ammonium acetate (cis-SMIA ammonium salt, CAS registration number is 97148- 39-5), control -30~10℃ and stir for 60-240min, react to obtain cis-methoxyiminofuran acetyl chloride solution (cis-SMIA acid chloride solution); wherein, the solvent includes but not limited to esters, alcohols, hydrocarbons ketones, aldehydes, amines, nitriles, ethers, etc.; amide reagents include but not limited to N,N-dimethylformamide, N,N-dimethylacetamide, etc.; phosphorus pentachloride and The mass ratio of the solvent is 1:2 to 12, that is, 1:2 to 1:12, the mass ratio of phosphorus pentachloride to solvent is preferably 1:3 to 8; the mass ratio of amide reagent to phosphorus pentachloride is 0.2 to 3:1, that is, 0.2:1 to 3:1; the mass ratio of phosphorus pent...
Embodiment 1
[0047] Preparation of trans-methoxyimidofuranacetyl chloride: Add 35g of phosphorus pentachloride to 180g of ethyl acetate, mix well, cool down to 0-10°C, add 50g of dimethylacetamide, then add 30g of cis-methoxyimide Ammonium furanacetate ammonium salt, stirred at 0-10°C for 150 minutes to prepare cis-methoxyimidofuranacetyl chloride, added 16g of acetone, then added 3.8g of 10% sulfuric acid solution, and stirred at 50°C for 6 hours to obtain trans Methoxyiminofuranacetyl chloride, purity 96.3%.
Embodiment 2
[0049] Preparation of trans-methoxyimidofuranacetyl chloride: Add 35g of phosphorus pentachloride into 230g of dichloromethane, stir to dissolve, cool down to -5~0°C, add 40g of dimethylformamide, and then add 25g of cis-formaldehyde Oxyiminofuran acetic acid ammonium salt, controlled at -5~-5°C and stirred for 130 minutes to prepare cis-methoxyimidofuranacetyl chloride, added 10g of ethanol, then added 5g of 15% sodium hydroxide solution, controlled at 60°C and stirred for reaction After 4 hours, trans-methoxyimidofuranacetyl chloride was obtained with a purity of 96.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com