Compound for treatment of metabolic diseases, preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
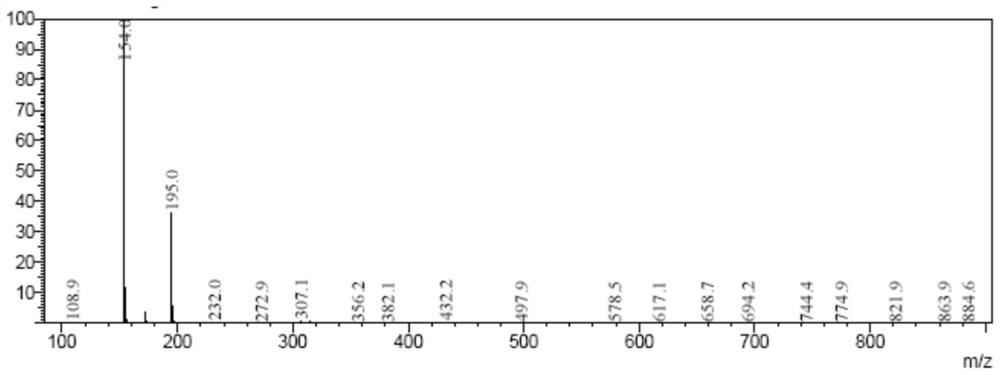
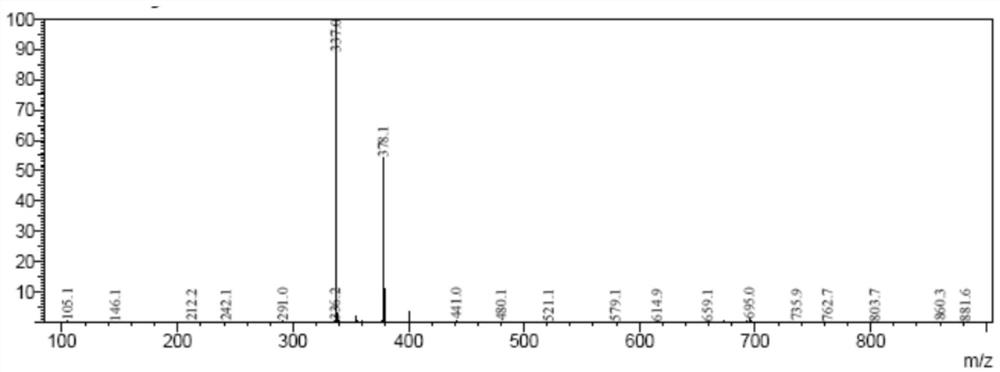
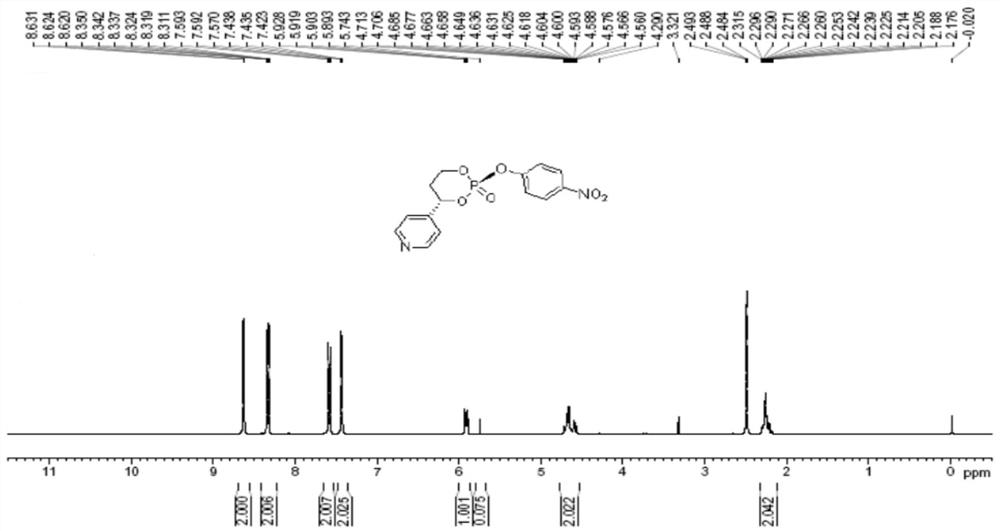
Image
Examples
Embodiment 1
[0098] Synthesis of embodiment 1 compound 1 (i.e. compound 1-H)
[0099] 1. Synthesis of Compound 2
[0100]
[0101] Add 300mL tetrahydrofuran to a 1000mL round bottom flask, then add 30g chenodeoxycholic acid (shown in formula a) (76.42mmol), and add 3mL perchloric acid, and add 100mL formic acid under stirring at room temperature. The temperature was controlled at 50°C and the reaction was stirred for 15 hours, then concentrated under reduced pressure to remove the solvent. The residue was diluted with 4 L of water, the solid was collected by filtration, and 2 L of dichloromethane was added to dissolve the solid. Anhydrous sodium sulfate was added to dry the solution, filtered and concentrated under reduced pressure. The crude product was slurried by adding 4 L of petroleum ether, and the white solid was collected by filtration to obtain 32.8 g of compound 2 with a yield of 98%.
[0102] 2. Synthesis of compound 3
[0103]
[0104] Under the protection of argon, a...
Embodiment 2
[0170] The synthesis of embodiment 2 compound 2-0
[0171] 1. Synthesis of compound 2-1
[0172]
[0173] A certain volume of anhydrous diethyl ether was added into the there-necked flask, then phenol (1000g, 1.0eq) and triethylamine (1070g, 1.0eq) were added, and phosphorus oxychloride (1600g, 1.0eq) was added dropwise with stirring at 0°C. Under the protection of nitrogen, react for 3h. It was then allowed to warm naturally to room temperature overnight. After the reaction, the triethylamine salt was quickly filtered off, and the filtrate was collected, concentrated, and dried to obtain 800 g of oily product 2-1, which was directly used in the next reaction without further purification.
[0174] 2. Synthesis of compound PH-MIK-001-21
[0175]
[0176]A certain volume of anhydrous dichloromethane 20L was added to the three-necked flask, and then triethylamine (770g, 2.0eq.) was added. The reaction system was placed at -78°C, and compound 2-1 (800g, 1.0 eq) A solutio...
Embodiment 3
[0222] 1. Synthesis of compound PH-MIK-001-22
[0223]
[0224] A certain volume of anhydrous dichloromethane 20L was added to the three-necked flask, and then triethylamine (770g, 2.0eq.) was added. The reaction system was placed at -78°C, and compound 2-1 (according to Example Prepared by the preparation method in 2, 800g, 1.0eq.) solution dissolved in anhydrous dichloromethane and (S)-3-(chloroamino)-1-isopropoxybutan-2-one (631g, 1.0eq.), reacted for 2h after the dropwise addition, and then allowed it to return to room temperature naturally and overnight. The solvent was removed, the residue was washed with ethyl acetate, and then filtered, and the filtrate was concentrated and dried to obtain compound PH-MIK-001-22 (760 g), which was directly used in the next reaction.
[0225] 2. Synthesis of compound 3-1
[0226]
[0227] The crude product 2-13 (120g, 1.0eq.) and N-methylimidazole (126g, 8.0eq.) were added to anhydrous tetrahydrofuran, and the compound PH-MIK- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com