Synthesis and application of novel functional material based on diazosulfide
A technology of benzothiadiazole and functional materials, applied in the direction of luminescent materials, liquid crystal materials, color-changing fluorescent materials, etc., can solve the problems of unreported complex liquid crystal phase and phase structure, and achieve high-efficiency luminous performance and liquid crystal performance, the effect of improving liquid crystal performance and solubility performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
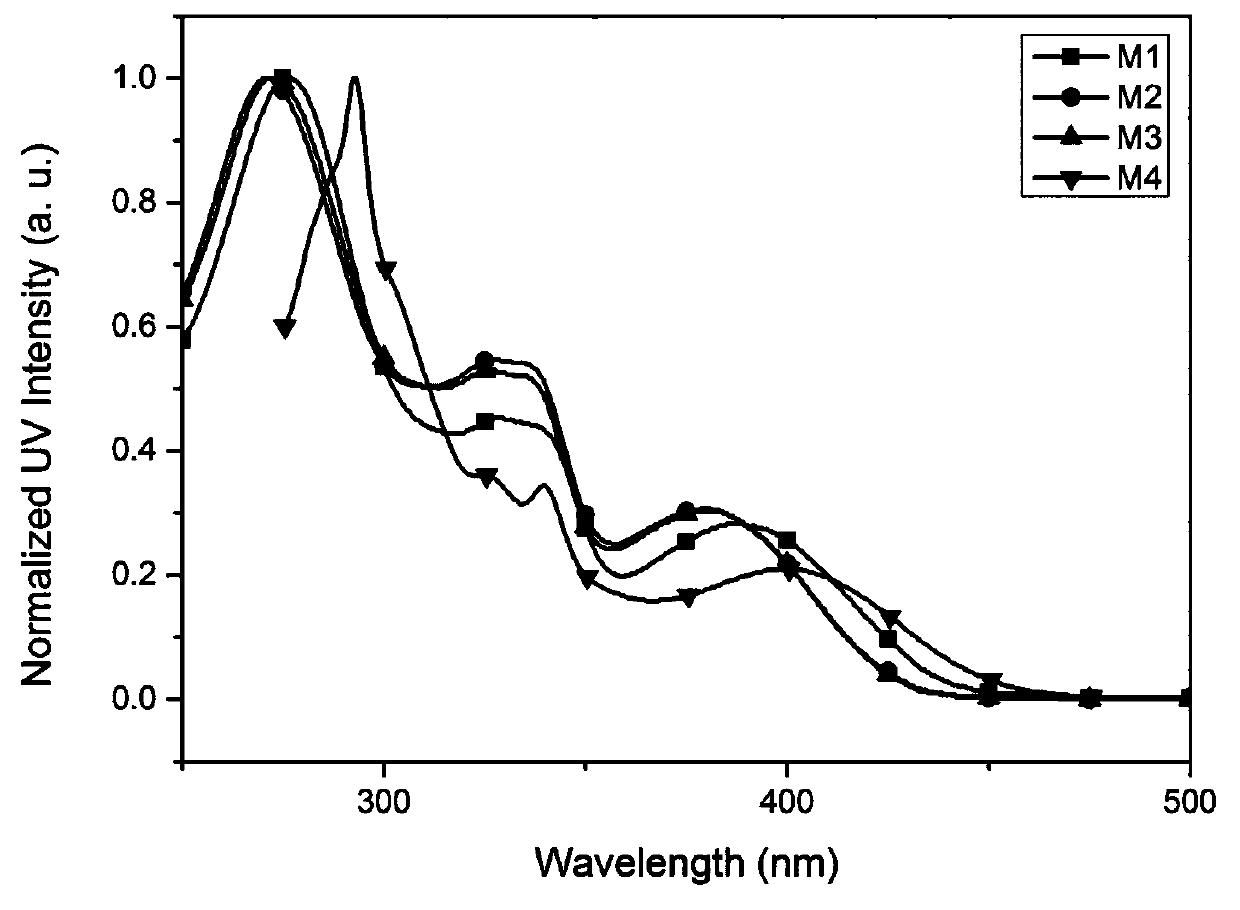
Image
Examples
Embodiment 1
[0020]
[0021] Under ice-cooling, add 4,7-dibromo-2,1,3-benzothiadiazole (6.0 g, 20.5 mmol) and H 2 SO 4 (12 mL), followed by slow dropwise addition of HNO 3 (24 mL). After reacting at room temperature for 6 hours, the reactant was poured into ice water, and suction filtered to obtain 5.12 g of a yellow solid (yield 73%). 1 H NMR (400 MHz, CDCl 3 ) δ 8.28 (s, 1H).
Embodiment 2
[0023]
[0024] Add 4,7-dibromo-2,1,3-benzothiadiazole-5-nitro (5.0 g, 14.7 mmol) and glacial acetic acid (120 mL) into a 200 mL two-necked flask, place in an ice bath, and separate Iron powder (4.2 g, 73.7 mmol) was added in batches. After reacting at room temperature for 12 hours, the reactant was poured into ice water, and suction filtered to obtain 4.3 g of a tan solid (95% yield). 1 H NMR (400 MHz, CDCl 3 ) δ 7.47 (s,1H), 4.64 (s, 2H).
Embodiment 3
[0026]
[0027]
[0028]
[0029]
[0030] Preparation of compound sm1
[0031] Add 4,7-dibromo-2,1,3-benzothiadiazol-5-amine (1.0 g, 3.24mmol), 4-tert-butylphenylboronic acid (1.0 g, 7.14mmol ), tetrakistriphenylphosphine palladium (187mg, 0.0162mmol), ethanol (10 mL), toluene (30 mL) and 2M potassium carbonate aqueous solution (10 mL), the mixture was refluxed under nitrogen for 24 hours. After the reactants were cooled to room temperature, the mixture was washed with CH 2 Cl 2 (3×30 mL) extraction; the organic layer was successively washed with water (60 mL), dried, and evaporated under reduced pressure to remove the solvent; the residue was column layered with petroleum ether:dichloromethane (V:V=1:2) as the eluent After separation and recrystallization, 430 mg of the target product was obtained (yield: 39%). 1 H NMR (300 MHz, CDCl 3 ) δ 7.86 (d, J = 8.4 Hz, 2H), 7.61-7.53 (m, 6H),7.28 (s, 1H), 4.25 (s, 2H), 1.40 (s, 19H).
[0032] Preparation of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com