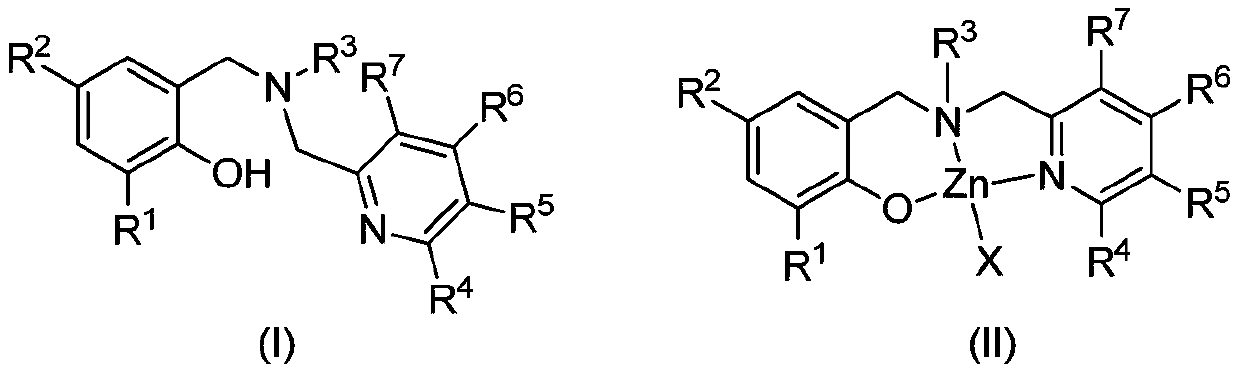
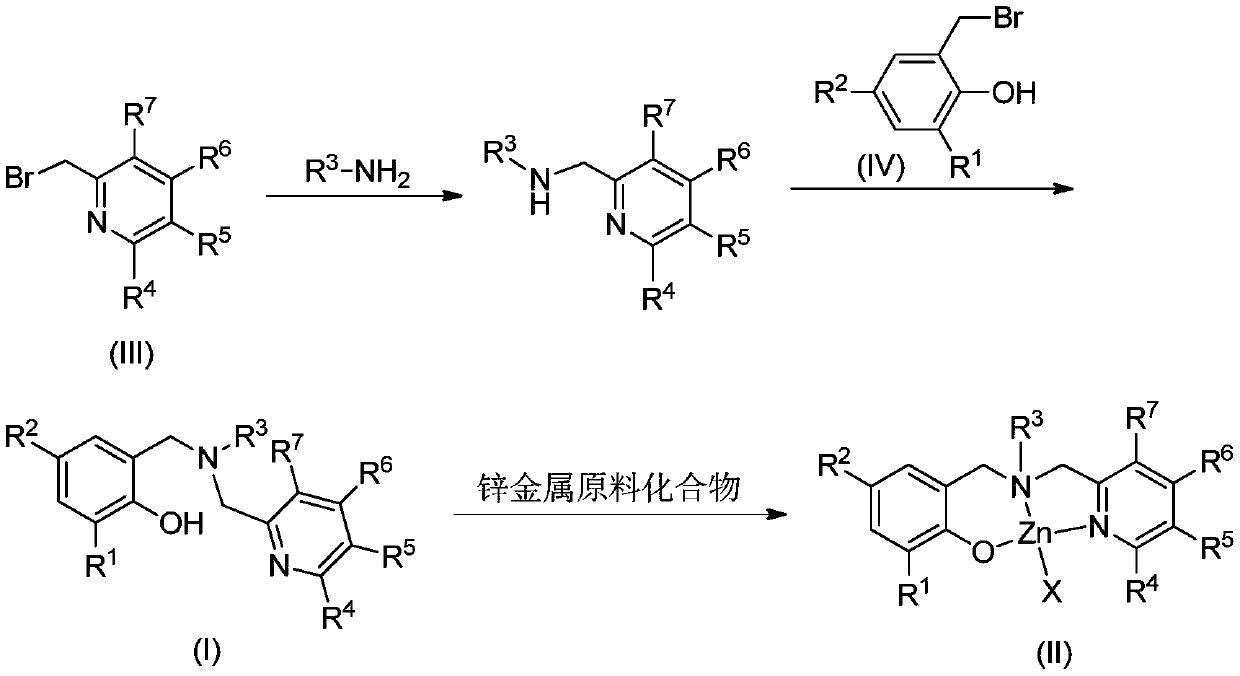
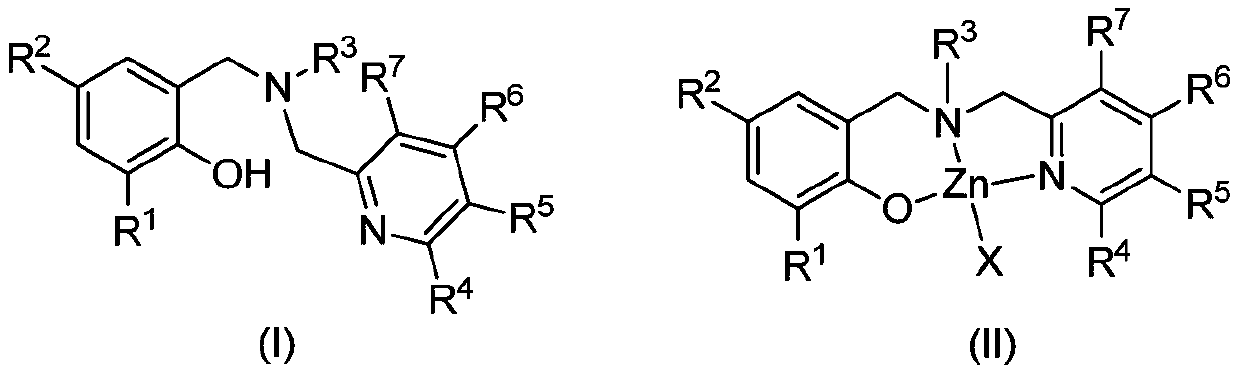
Aminophenolate zinc complex containing pyridine ring as well as preparation method and application thereof
An aminophenoxy zinc, pyridine ring technology, applied in zinc organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low catalyst activity, minimal catalyst, poor activity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of Ligand L1
[0053] (1) Synthesis of N-benzyl-1-(6-methylpyridin-2-yl)methanamine
[0054]
[0055] Add benzylamine (16.07g, 150mmol) and potassium carbonate (1.66g, 12mmol) in the reaction flask, 2-bromomethyl-6-picoline (2.48g, 10mmol) and 25mL N,N-dimethylformamide Afterwards, react for 12 hours. After column chromatography, a red-brown oily liquid (1.02 g, 48%) was obtained.
[0056] (2) Synthesis of Ligand L1
[0057] N-benzyl-1-(6-methylpyridin-2-yl)methanamine (1.22g, 5.73mmol), potassium carbonate (0.95g, 6.87mmol), 2-bromomethyl-4- Methyl-6-tritylphenol (2.54g, 5.73mmol) and 20mL N,N-dimethylformamide were reacted at room temperature for 8 hours. Add water to quench the reaction, extract with ethyl acetate, combine the organic phases, dry over anhydrous magnesium sulfate, filter, evaporate the solvent under reduced pressure, and recrystallize with dichloromethane and petroleum ether to obtain white solid L1 (2.72g, 83%).
[0058]
[0059]...
Embodiment 2
[0061] Synthesis of Ligand L2:
[0062] (1) Synthesis of N-[(6-methylpyridin-2-yl)methyl]n-hexylamine
[0063]
[0064] Except that n-hexylamine (30.36g, 300mmol), potassium carbonate (3.32g, 24mmol) and 2-bromomethyl-6-picoline (3.72g, 20mmol) were used as raw materials, other operating steps were the same as in Example 1. After column chromatography, a red-brown oil (2.03g, 49%) was obtained.
[0065] (2) Synthesis of Ligand L2
[0066] N-[(6-methylpyridin-2-yl)methyl]n-hexylamine (1.10g, 5.35mmol), potassium carbonate (0.89g, 6.42mmol) and 2-bromomethyl-4-methyl -Except for 6-tritylphenol (2.37g, 5.35mmol), other operating steps are the same as in Example 1. Recrystallization from dichloromethane and petroleum ether gave white solid L2 (2.39 g, 79%).
[0067]
[0068] 1 H NMR (CDCl 3 ,400MHz,298K):δ10.67(br s,1H,OH),7.35(dd,1H, 3 J=7.6Hz, 3 J=7.6Hz, PyH), 7.25-7.09(m, 15H, ArH), 6.96(d, 1H, 3 J=7.6Hz,PyH),6.88(d,1H, 4 J=1.6Hz, ArH), 6.80(d, 1H, 4 J=1.6Hz, ArH...
Embodiment 3
[0070] Synthesis of Ligand L3:
[0071] (1) Synthesis of N-[(6-methylpyridin-2-yl)methyl]cyclohexylamine
[0072]
[0073] Except that cyclohexylamine (29.75g, 300mmol), potassium carbonate (3.32g, 24mmol) and 2-bromomethyl-6-picoline (3.72g, 20mmol) were used as raw materials, other operating steps were the same as in Example 1. After column chromatography, a red-brown oil (1.99 g, 48%) was obtained.
[0074] (2) Synthesis of Ligand L3
[0075] N-[(6-methylpyridin-2-yl)methyl]cyclohexylamine (1.55g, 7.59mmol), potassium carbonate (1.27g, 9.14mmol) and 2-bromomethyl-4-methyl Base-6-tritylphenol (3.36g, 7.59mmol), other operating steps are the same as in Example 1. Recrystallization from dichloromethane and petroleum ether gave white solid L3 (3.39 g, 79%).
[0076]
[0077] 1 H NMR (CDCl 3 ,400MHz,298K):δ10.85(br s,1H,OH),7.33(dd,1H, 3 J=7.6Hz, 3 J=7.6Hz, PyH), 7.24-7.09(m, 15H, ArH), 6.93(d, 1H, 3 J=7.6Hz,PyH),6.85(d,1H, 4 J=1.6Hz, ArH), 6.76(d, 1H, 4 J=1.6Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com