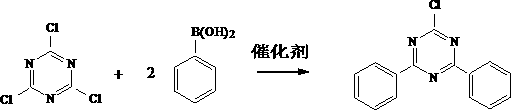
Preparation method of 2-chlorine-4,6-diphenyl-1,3,5-triazine
A technology of diphenyl and phenylboronic acid, applied in the direction of organic chemistry, can solve the problems of lack of economic attractiveness, corrosion equipment, long synthesis route, etc., and achieve the effects of avoiding the pressure of three wastes treatment, mild reaction conditions, and overcoming poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 18.5g (0.1mol) 1,3,5-triazine, 24.4g (0.2mol) phenylboronic acid, 21.2g (0.2mol) sodium carbonate, 1.2g catalyst Ni(dppf)Cl into a 500ml reaction flask 2 , 50g of toluene, stirring to heat up to 80°C, heat preservation reaction for 8h, cooling to room temperature, adding 100ml of water dropwise to quench the reaction, then heating up and distilling to recover the solvent toluene, cooling and filtering after no toluene fractionation, washing the filter cake with water until neutral, to obtain 2-Chloro-4,6-diphenyl-1,3,5-triazine 24.4g, yield 91.3%, purity 98.5%, of which the monosubstitution content is 0.48%, and the trisubstitution content is 0.37%.
Embodiment 2
[0023] Add 18.5g (0.1mol) 1,3,5-triazine, 26.8g (0.22mol) phenylboronic acid, 34.5g (0.25mol) potassium carbonate, 1g catalyst Ni(pcy 3 ) 2 Cl 2 , 100g tetrahydrofuran, stir and heat up to 70°C, heat-retain for 12 hours, cool to room temperature, add 100ml of water dropwise to quench the reaction, then heat up and distill to recover solvent tetrahydrofuran, cool down and filter after no tetrahydrofuran is fractionated, wash the filter cake with water until neutral, and obtain 25.2 g of 2-chloro-4,6-diphenyl-1,3,5-triazine, the yield is 94.1%, the purity is 98.9%, the content of mono-substitution is 0.28%, and the content of tri-substitution is 0.56%.
Embodiment 3
[0025] Add 18.5g (0.1mol) 1,3,5-triazine, 23.2g (0.19mol) phenylboronic acid, 31.8g (0.3mol) sodium carbonate, 2g catalyst Ni (pph 3 ) 2 Cl 2 , 100g of toluene, stirring to heat up to 110°C, heat preservation reaction for 4h, cooling to room temperature, adding 100ml of water dropwise to quench the reaction, then heating up and distilling to recover the solvent toluene, cooling and filtering after no toluene fractionation, washing the filter cake with water until neutral, to obtain 24.8g of 2-chloro-4,6-diphenyl-1,3,5-triazine, the yield is 92.6%, the purity is 98.3%, the content of mono-substitution is 0.68%, and the content of tri-substitution is 0.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com