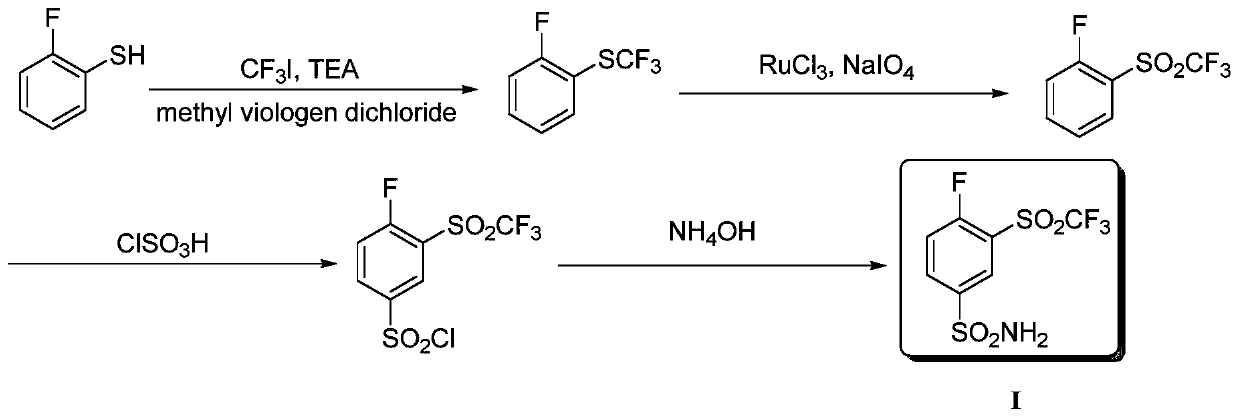
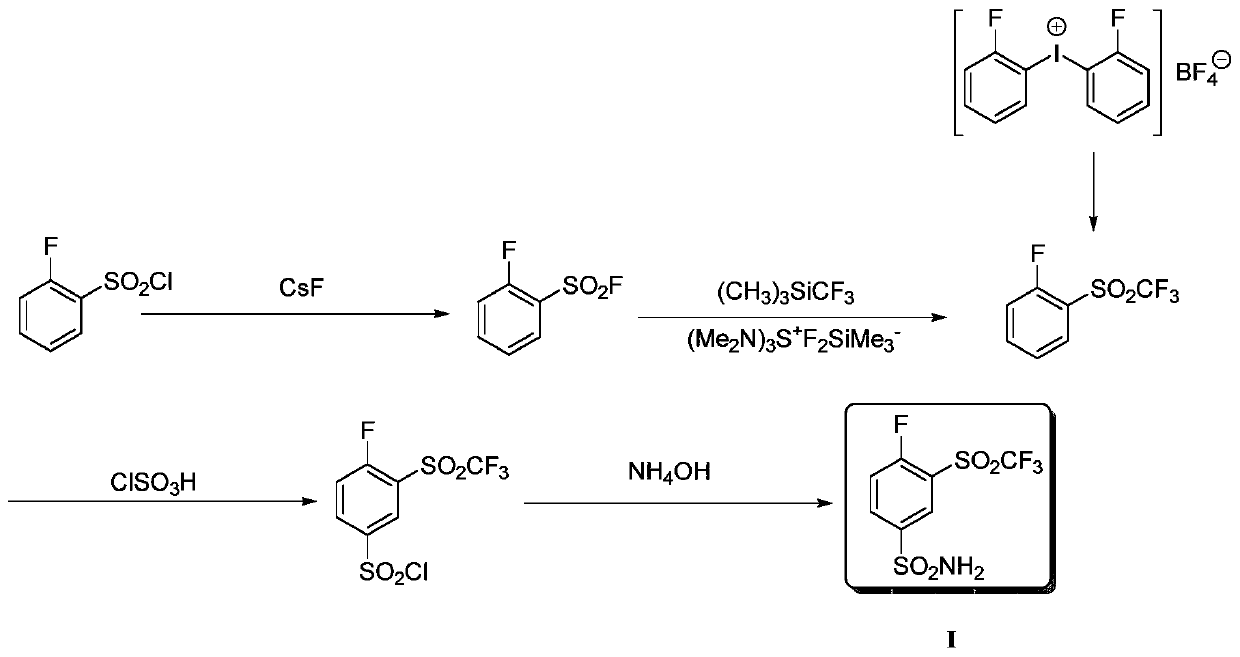
Method for producing 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide
A technology of trifluoromethanesulfonyl and benzenesulfonamide, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high price of trifluoroiodomethane, harsh reaction conditions, and low reaction yield , to achieve broad commercial application prospects, mild and controllable reaction conditions, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
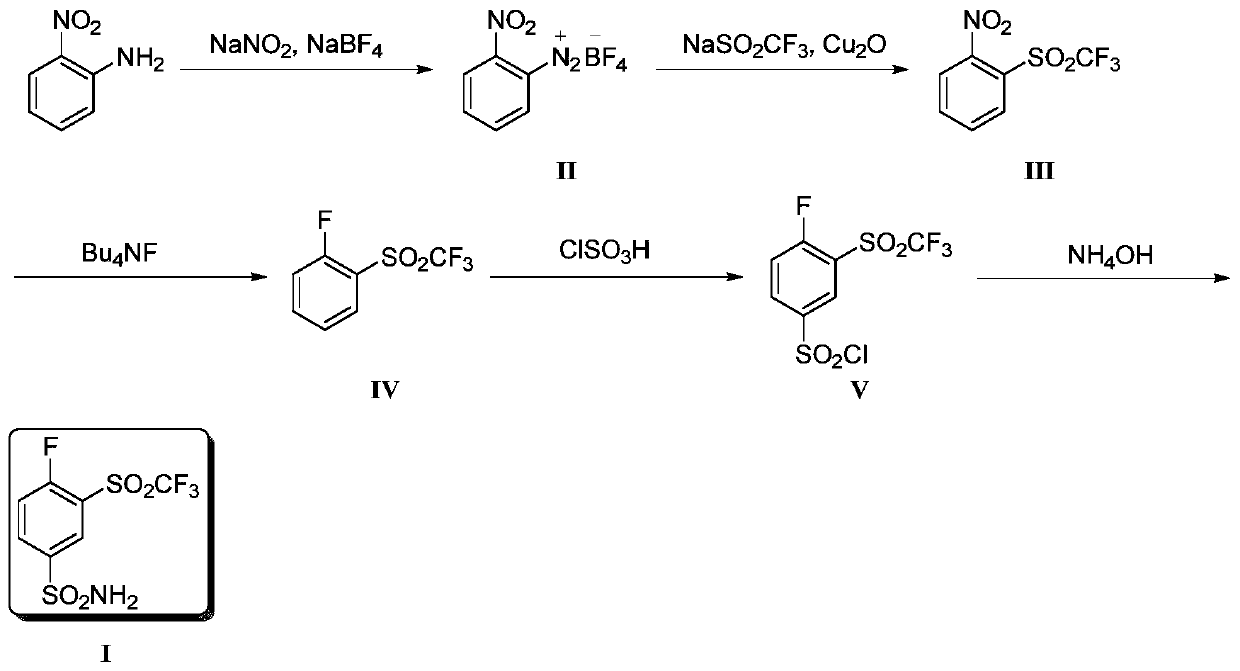
[0035] Synthesis of step (1) compound (II)
[0036] After mixing 2-nitroaniline (250g, 1.81mol) with concentrated hydrochloric acid (450mL) and water (365mL), add sodium nitrite (125g, 1.81mol) and water (300 mL) dropwise to the above solution at 0°C ) mixed solution. After the dropwise addition was completed, stirring was continued for 1 h. Subsequently, a mixed solution of sodium fluoroborate (505 g, 4.6 mol) and water (900 mL) was continuously added dropwise at this temperature, and stirred for 1 h after the dropwise addition was completed. The obtained solution was filtered, and the filter cake was washed three times with ice water (100 g) and methanol (100 mL), respectively, and 363 g of yellow solid II were obtained after drying, with a yield of 85%.
[0037] Synthesis of step (2) compound (III)
[0038] Sodium trifluoromethanesulfinate (396 g, 2.53 mol), cuprous oxide (12 g, 0.08 mol) and DMSO (4 L) were successively added into the three-necked flask. When the tempe...
Embodiment 2
[0046] Step (1) is the same as step (1) in embodiment 1
[0047] Synthesis of step (2) compound (III)
[0048] Sodium trifluoromethylsulfinate (3.96 g, 25.3 mmol), cuprous thiophene-2-carboxylate (CuTC) (160 mg, 0.84 mmol) and DMF (40 mL) were sequentially added into the three-necked flask. When the temperature of the reaction solution was lowered to 15° C., diazonium salt compound II (2 g, 8.4 mmol) was added in batches. After continuing to react for 4 hours, the reaction solution was poured into ice water (80 g), extracted with dichloromethane, the organic phase was washed with water, washed with brine, dried over sodium sulfate, concentrated under reduced pressure and then subjected to column chromatography to obtain 835 mg of light yellow solid III. Yield 39%. 1 The H NMR data is consistent with step (2) in Example 1.
[0049] Synthesis of step (3) compound (IV)
[0050] Compound III (1.55 g, 6.1 mmol), KF (887.4 mg, 15.3 mmol) and DMSO (15 mL) were mixed and stirred a...
Embodiment 3
[0053] Step (1) is the same as step (1) in embodiment 1
[0054] Synthesis of step (2) compound (III)
[0055] Add sodium trifluoromethanesulfinate (3.96g, 25.3mmol), CuBF successively into the three-necked flask 4 (160 mg, 0.84 mmol) and DMSO (50 mL). When the temperature of the reaction solution was raised to 60° C., diazonium salt compound II (2 g, 8.4 mmol) was added in batches. After continuing to react for 4 hours, the reaction solution was poured into ice water (100 g), added dichloromethane for extraction, the organic phase was washed with water, washed with salt water, dried over sodium sulfate, concentrated under reduced pressure and then subjected to column chromatography to obtain 386 mg of light yellow solid III, Yield 18%. 1 The H NMR data is consistent with step (2) in Example 1.
[0056] Synthesis of step (3) compound (IV)
[0057] Compound III (1.55 g, 6.1 mmol), CsF (2.32 g, 15.3 mmol) and DMF (15 mL) were mixed and stirred at 100°C. The reaction was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com