Positive electrode active material for zinc-bromine single-flow battery and preparation and application thereof
A positive electrode active material and liquid flow battery technology, applied in battery electrodes, regenerative fuel cells, circuits, etc., can solve the problems of aggravated positive electrode corrosion and high concentration of bromine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Potassium bromide and cuprous chloride were blended with deionized water at 50 degrees Celsius at a molar ratio of 2:1, and the concentration of bromide ions was 4M. Stir evenly, and the reaction time is 4 hours; after the reaction, centrifuge and dry at 80 degrees Celsius, see figure 1 , the material is spherical with a uniform particle size of 500-600nm.
[0032] The prepared cuprous bromide and glucose were blended in a molar ratio of 1:0.5, stirred at 5000 rpm by a high-speed ball mill for 1 hour, and then taken out.
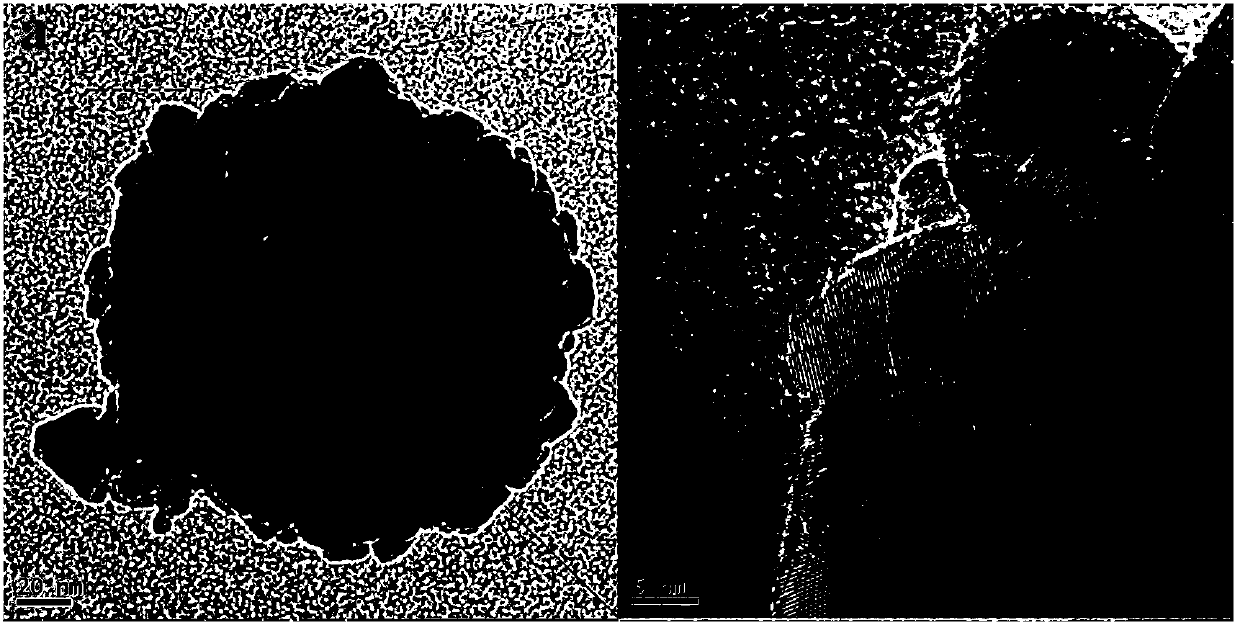
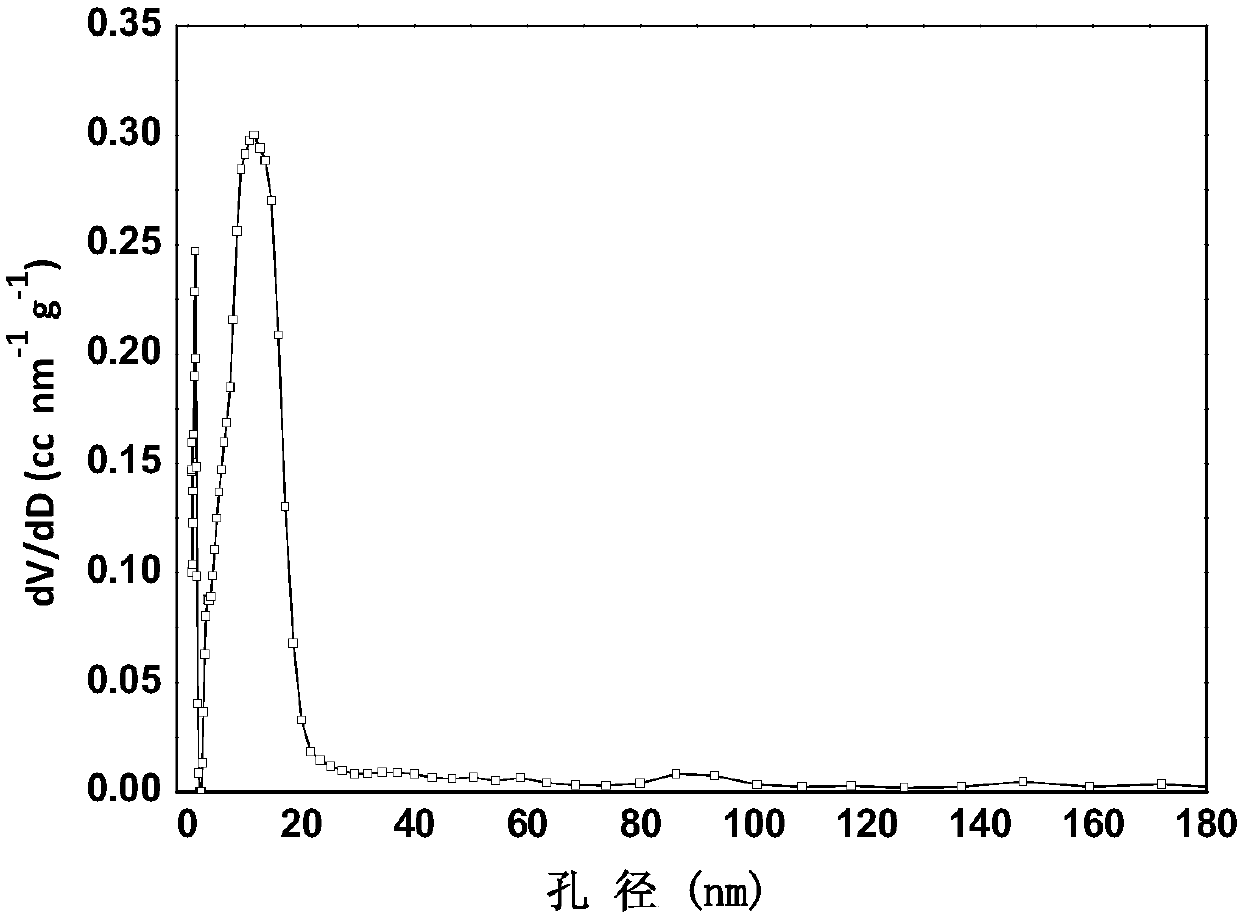
[0033] The above materials are sintered in an inert atmosphere of argon, the sintering temperature is 1000 degrees Celsius, the heating rate is 10 degrees Celsius / min, the sintering temperature time is 1h, and then the carbon-coated cuprous bromide material is obtained, see figure 2 . It can be seen that there is a uniform carbon coating layer on the surface of the material, and the BET and pore size distribution of the carbon coating layer are sho...
Embodiment 2
[0040] Sodium bromide and cuprous sulfate are blended with deionized water at 30 degrees Celsius at a molar ratio of 4:1, and the concentration of bromide ions is 8M. Stir evenly, and the reaction time is 10 hours; after the reaction, centrifuge and dry at 80 degrees Celsius. The material is spherical with a uniform particle size of 500-600nm.
[0041] The prepared cuprous bromide and glucose were blended according to the molar ratio of 1:1, stirred at 10,000 rpm by a high-speed ball mill for 4 hours, and then taken out.
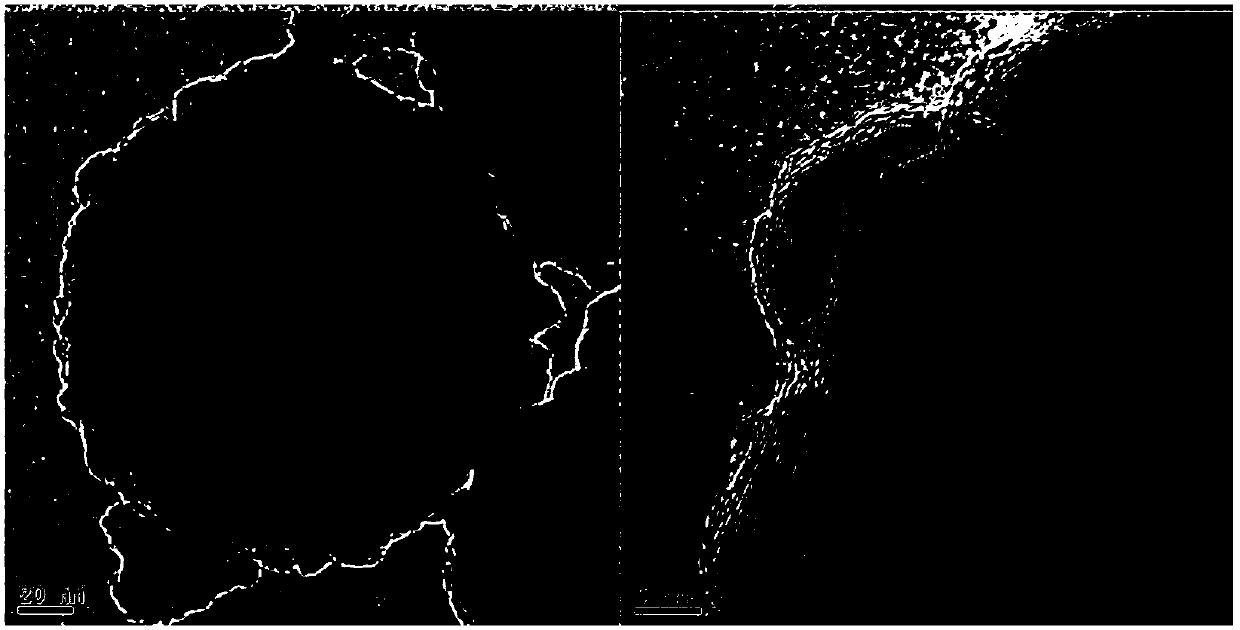
[0042] Place the above material in an inert atmosphere of nitrogen for sintering, the sintering temperature is 500°C, the heating rate is 1°C / min, and the sintering temperature time is 4h, then take out the carbon-coated cuprous bromide material, see Figure 4 , it can be seen that unlike Example 1, the material prepared in Example 2 is a regular and compact spherical shape.
Embodiment 3
[0044] Blend 1:1 potassium bromide and sodium bromide with 1:1 cuprous sulfate and cuprous chloride at a molar ratio of 3:1 with deionized water at 40 degrees Celsius, with a bromide ion concentration of 6M. Stir evenly, and the reaction time is 5 hours; after the reaction, centrifuge and dry at 80°C. The material is in the shape of a rod with a uniform particle size of 900-1000nm.
[0045] The prepared cuprous bromide and glucose were blended in a molar ratio of 1:0.75, stirred at 7500 rpm by a high-speed ball mill for 2 hours, and then taken out.
[0046] The above materials are sintered in an inert atmosphere of argon, the sintering temperature is 700 degrees Celsius, the heating rate is 5 degrees Celsius / minute, the sintering temperature time is 2h, and then the carbon-coated cuprous bromide material is obtained, see Figure 5 , it can be seen that the material synthesized in Example 3 is rod-shaped.
[0047] As can be seen from the above examples, the structure and morpho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com