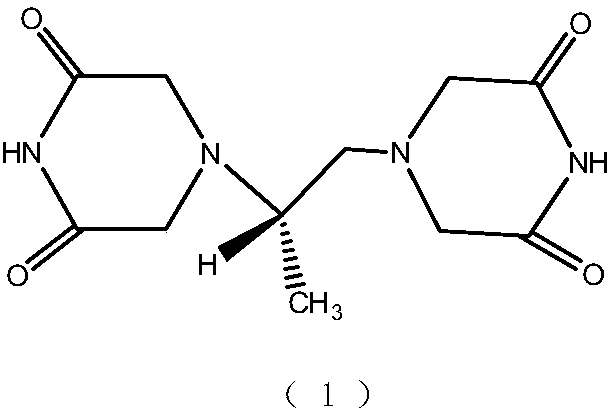
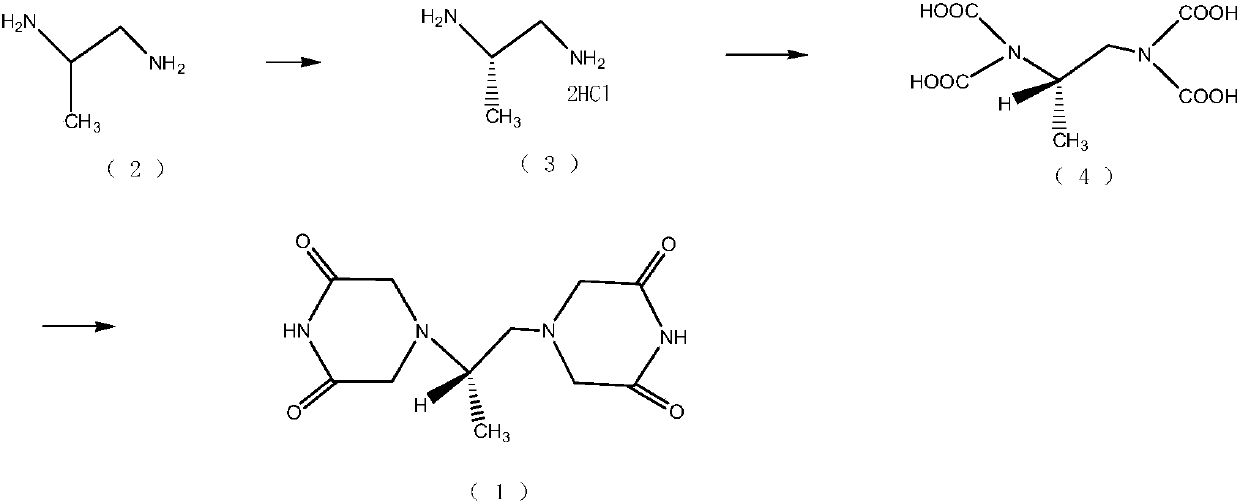
Dexrazoxane preparation method
A technology of dexrazoxane and reaction is applied in the field of preparation of dexrazoxane, which can solve the problems of tediousness, high price and high cost, and achieve the effects of simple reaction steps, good product quality and convenient post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0017] Preparation of (S)-1,2-propanediamine dihydrochloride (3): Add 30.0g D-(-)-tartaric acid and 105mL water and 8.0g (±)-1,2- Propylenediamine, stirred to dissolve, cooled, added dropwise, heated to reflux for 2 hours with stirring. Stop stirring, first raise the temperature to 80°C, keep it warm for 1 hour, then gradually lower the temperature to room temperature, filter with suction, and dry in vacuum to obtain 16.1 g of (S)-1,2-propanediamine bitartrate. Add 16.1g (S)-1,2-propanediamine bitartrate and 150mL water into the reaction flask, heat to dissolve, then add a solution made of 7.43g potassium chloride and 20mL water, and keep stirring at 70°C for 2 Hour. Cool, and let stand in the refrigerator for crystallization. After suction filtration, the filtrate was distilled to dryness under reduced pressure to obtain 5.1 g of yellow solid (3), with a yield of 84%, [α]2 OD=-4.02° (C=1%, H2O).
[0018] (S)-N, N, N', N'-1, the preparation of 2-propanediaminetetraacetic ac...
Embodiment example 2
[0021] Preparation of (S)-1,2-propanediamine dihydrochloride (3): Add 50.0g D-(-)-tartaric acid and 105mL water and 8.0g (±)-1,2- Propylenediamine, stirred to dissolve, cooled, added dropwise, heated to reflux for 4 hours with stirring. Stirring was stopped, the temperature was slowly lowered to room temperature, suction filtered, and vacuum-dried to obtain 17.1 g of (S)-1,2-propanediamine bitartrate. Add 17.1g (S)-1,2-propanediamine bitartrate and 150mL water into the reaction flask, heat to dissolve, then add a solution made of 7.96g potassium chloride and 20mL water, keep stirring at 70°C 2 hours. Cool, and let stand in the refrigerator for crystallization. After suction filtration, the filtrate was distilled to dryness under reduced pressure to obtain 4.3 g of yellow solid (3), with a yield of 75%, [α]2 OD=-4.02° (C=1%, H2O).
[0022] (S)-N, N, N', N'-1, the preparation of 2-propanediaminetetraacetic acid (4): add 9.2g chloroacetic acid 90mL water successively in reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com