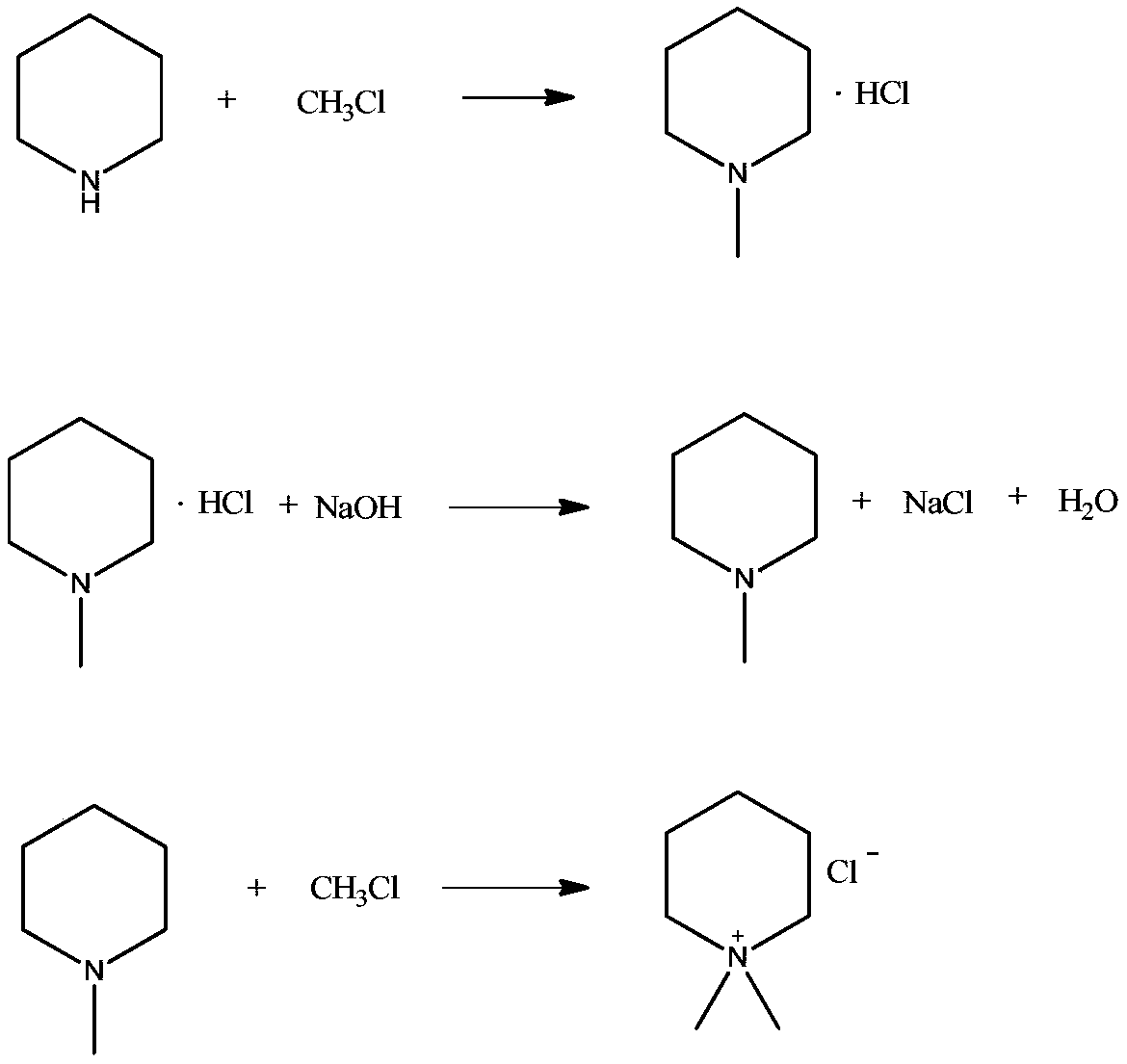
Preparation method of N, N-dimethyl piperidine chloride salt
A technology of dimethylpiperidine and chloride salt, which is applied in the field of preparation of N,N-dimethylpiperidine chloride salt, can solve the problems of poor operation safety, low product content, and low yield, and achieve low cost, High yield and content, safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the preparation of N,N-dimethyl piperidine chloride salt
[0025] Take 80g of 25% NaOH solution, 160g of n-butanol, and 40g of piperidine into a 500mL autoclave in sequence. After sealing it, put 55g of methyl chloride into it, react at 70°C for 4h, release the material and transfer it to a 500mL distillation bottle, and adjust the vacuum to - 0.07MPa, heat up to 70°C to evaporate 43g of water, then adjust the temperature to 60°C for filtration, filter out sodium chloride, transfer the filtrate to a 500mL pear-shaped flask, and use rotary thin-film evaporation at -0.09MPa, 85°C The device evaporates the material to dryness. The temperature was lowered to 25° C. to obtain 69 g of product with a content of 99.6% and a yield of 98.7%.
Embodiment 2
[0026] Embodiment 2, the preparation of N,N-dimethyl piperidine chloride salt
[0027] Take 95g of 20% NaOH solution, 120g of isobutanol, and 40g of piperidine into a 500mL autoclave in sequence, and then put 55g of methyl chloride into the autoclave after sealing, react at 65°C for 5h, release the material and transfer it to a 500mL distillation bottle, and adjust the vacuum to - 0.06MPa, heat up to 70°C to evaporate 64g of water, then adjust the temperature to 50°C for filtration, filter out sodium chloride, transfer the filtrate to a 500mL pear-shaped flask, and use rotary thin-film evaporation at -0.09MPa, 75°C The device evaporates the material to dryness. The temperature was lowered to 25° C. to obtain 69.5 g of product with a content of 99.3% and a yield of 99.1%.
Embodiment 3
[0028] Embodiment 3, the preparation of N,N-dimethyl piperidine chloride salt
[0029] Take 65g of 30% NaOH solution, 200g of n-butanol, and 40g of piperidine into a 500mL autoclave in sequence, and after sealing it, put 55g of methyl chloride into it, react at 60°C for 5h, release the material and transfer it to a 500mL distillation bottle, and adjust the vacuum to - 0.07MPa, heat up to 70°C to evaporate 30g of water, then adjust the temperature to 60°C for filtration, filter out sodium chloride, transfer the filtrate to a 500mL pear-shaped bottle, and use rotary thin-film evaporation at -0.09MPa, 85°C The device evaporates the material to dryness. The temperature was lowered to 25° C. to obtain 69.2 g of product with a content of 99.1% and a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com