9-benzyl naphthylamine and synthesis method thereof
A technology of benzylnaphthylamine and its synthesis method, which is applied in the field of organic synthesis, can solve the problems of cumbersome synthesis route of benzylnaphthylamine, difficulty in accurate positioning, and many by-products, and achieve expansion of imagination space, strong positioning effect, and simple steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
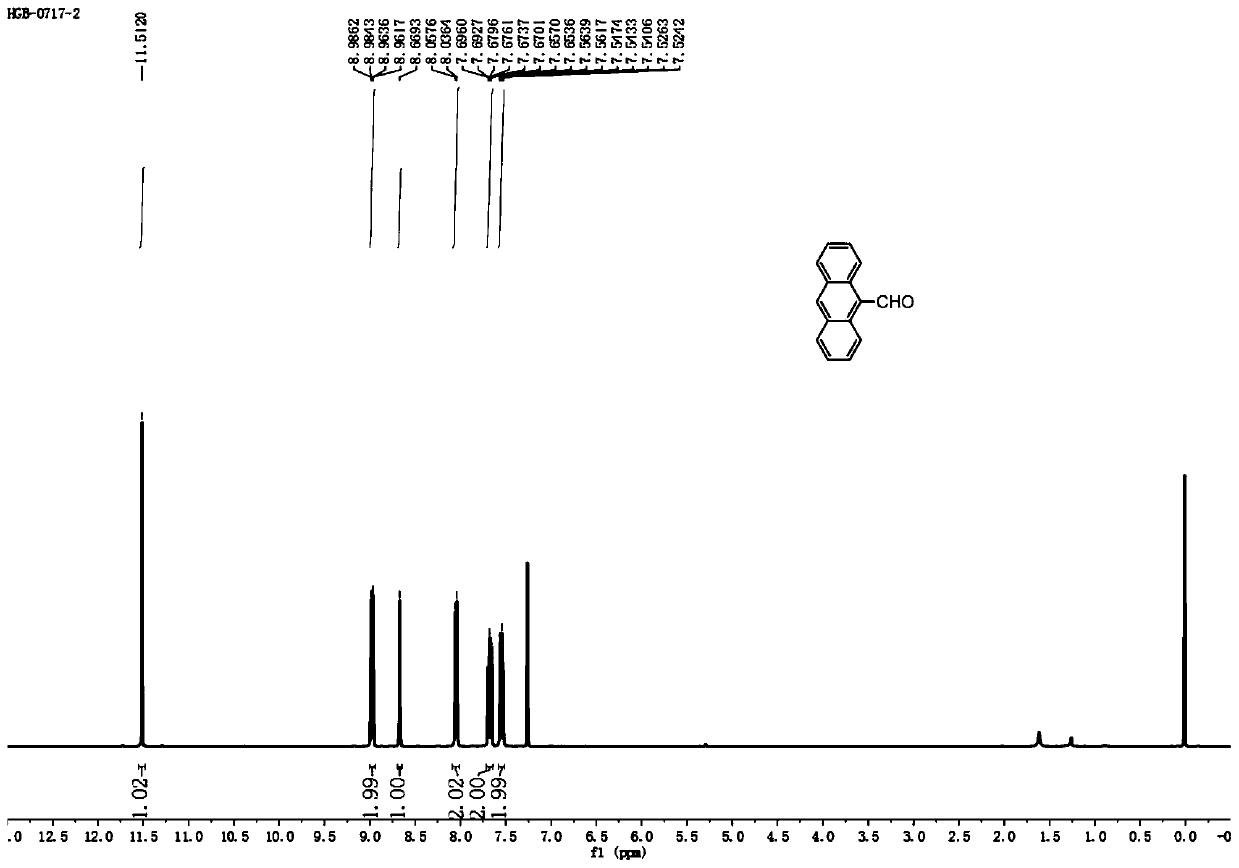
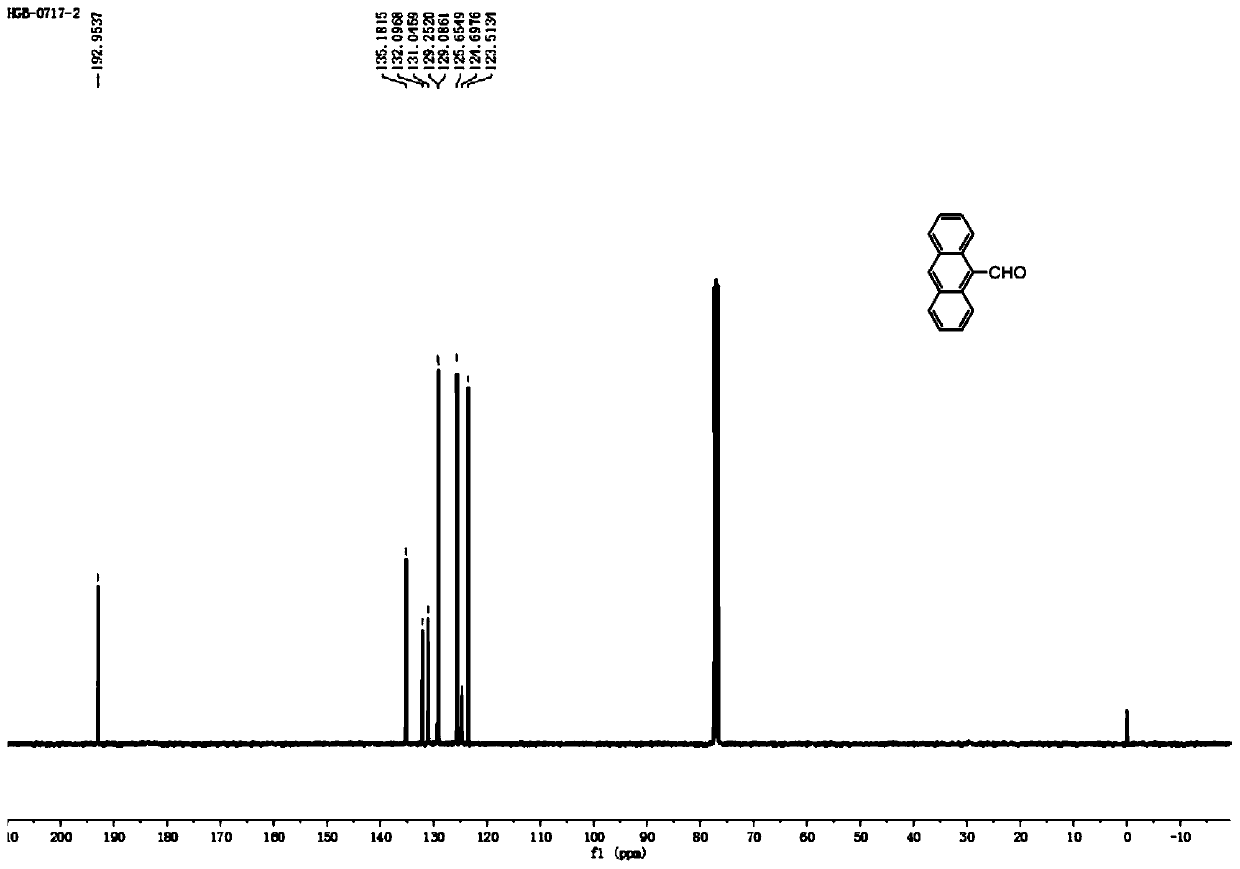
[0028] Step 1: Dissolve (2mmol, 356mg) anthracene in 10mL dichloromethane, cool down to 0°C, add (2mmol, 230mg) 1,1-dichloromethyl ether and 390mg titanium tetrachloride, warm up to room temperature, stir for 2 -5h, add 20mL of saturated sodium bicarbonate, extract with 20mL of dichloromethane to obtain an organic phase and dry it, evaporate the solvent under reduced pressure to obtain intermediate S1, namely 9-formylnaphthalene. As a yellow solid, the yield is 95%. m.p:135.1-136.0℃. 1 H NMR (400MHz, CDCl 3 )δ11.5(s,1H),8.97(dd,J=9.0,0.8Hz,2H),8.67(s,1H),8.05(d,J=8.5Hz,2H),7.70-7.65(m,2H ),7.61-7.47(m,2H)ppm; 13 C NMR (100MHz, CDCl 3 )δ193.0, 135.2, 132.1, 131.1, 129.3, 129.1, 125.7, 124.7, 123.5ppm; Intermediate S1 1 See the H NMR chart for details figure 2 , 13 See the C NMR chart for details image 3 .
[0029] Step 2: Intermediate S1 (2mmol, 412mg) and (2mmol, 234mg) tert-butyl carbamate obtained in step 1 were dissolved in a mixed solvent (10mL) of acetonitrile ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com