A kind of 9-benzylnaphthylamine and its synthetic method
A synthesis method and technology of benzylnaphthylamine, applied in the field of organic synthesis, can solve the problems of cumbersome synthesis route of benzylnaphthylamine, difficulty in accurate positioning, many by-products, etc., and achieve expansion of imagination space, strong positioning effect, and simple steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
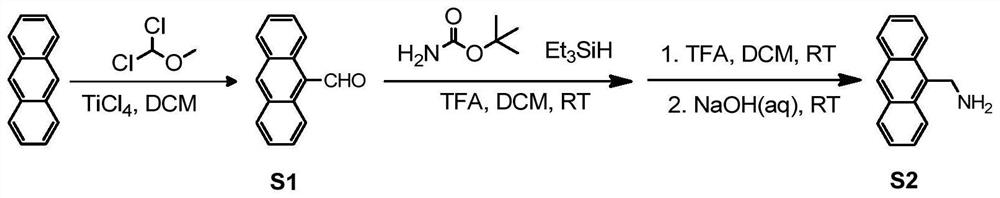
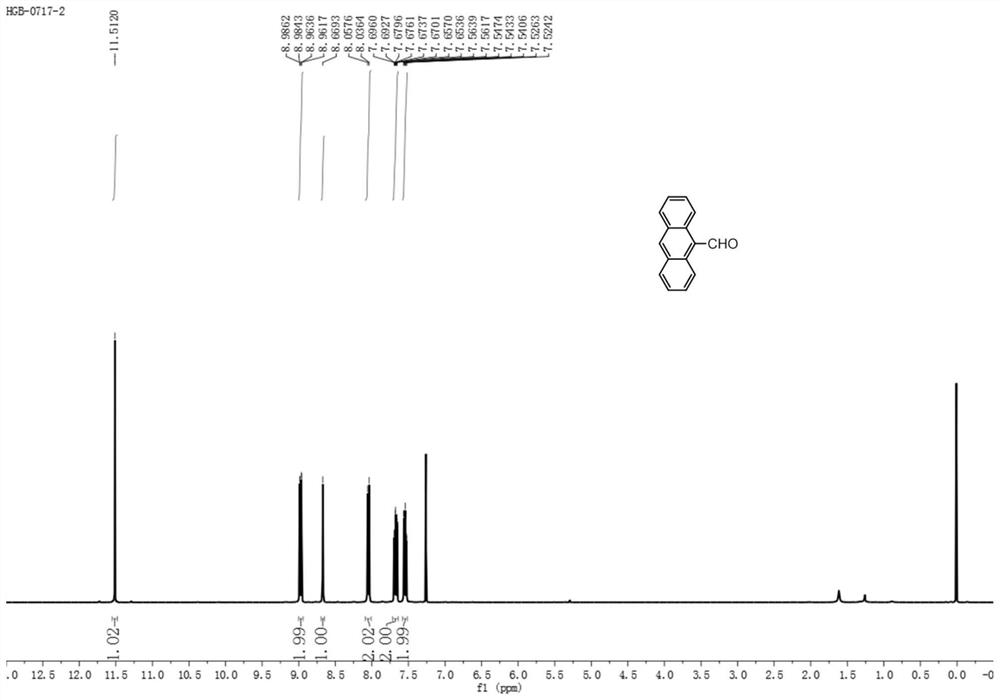
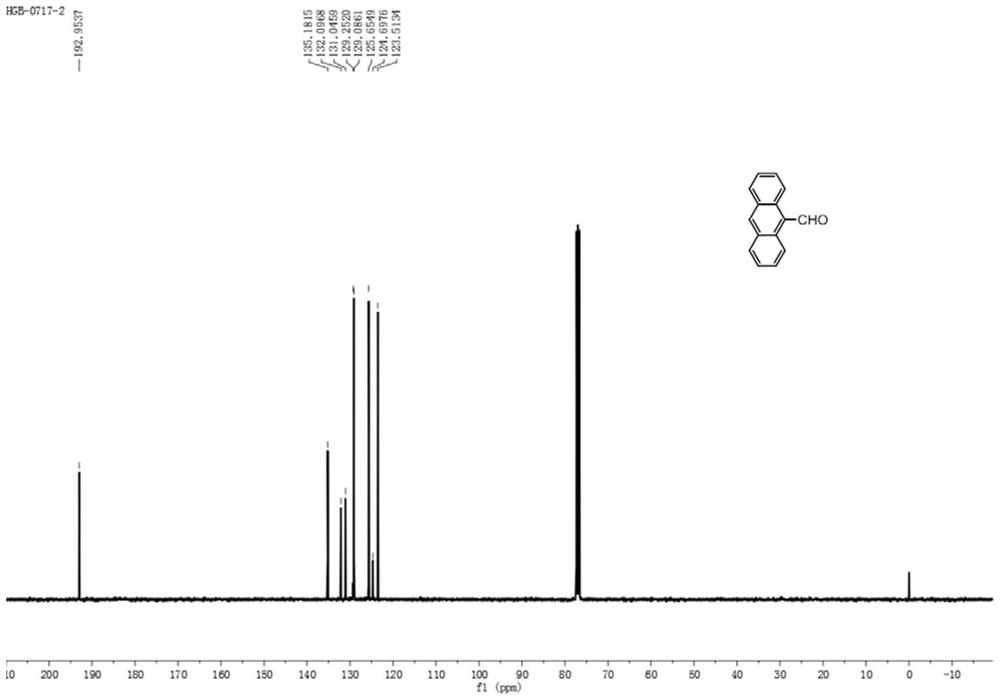
[0028] Step 1: Dissolve (2mmol, 356mg) anthracene in 10mL dichloromethane, cool down to 0°C, add (2mmol, 230mg) 1,1-dichloromethyl ether and 390mg titanium tetrachloride, warm up to room temperature, stir for 2 -5h, add 20mL of saturated sodium bicarbonate, extract with 20mL of dichloromethane to obtain an organic phase and dry it, evaporate the solvent under reduced pressure to obtain intermediate S1, namely 9-formylnaphthalene. As a yellow solid, the yield is 95%. m.p:135.1-136.0℃. 1 H NMR (400MHz, CDCl 3 )δ11.5(s,1H),8.97(dd,J=9.0,0.8Hz,2H),8.67(s,1H),8.05(d,J=8.5Hz,2H),7.70-7.65(m,2H ),7.61-7.47(m,2H)ppm; 13 C NMR (100MHz, CDCl 3 )δ193.0, 135.2, 132.1, 131.1, 129.3, 129.1, 125.7, 124.7, 123.5ppm; Intermediate S1 1 See the H NMR chart for details figure 2 , 13 See the C NMR chart for details image 3 .
[0029] Step 2: Intermediate S1 (2mmol, 412mg) and (2mmol, 234mg) tert-butyl carbamate obtained in step 1 were dissolved in a mixed solvent (10mL) of acetonitrile ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com