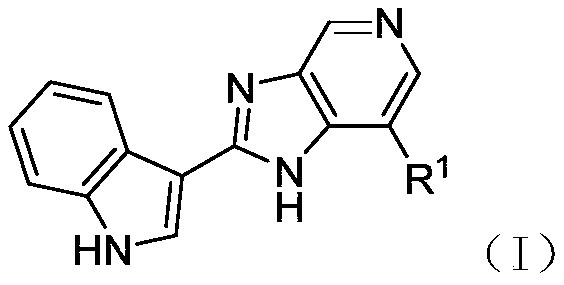
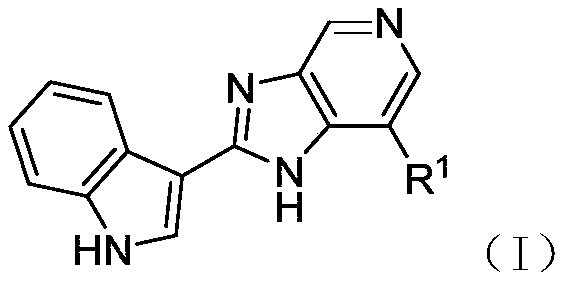
2-(indol-3-yl)-pyridinoimidazole and application thereof
A pyridoimidazole and methyl pyridyl technology, applied in the field of 2--pyridimidazole compounds, can solve the problems of large toxic and side effects, easy generation of drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
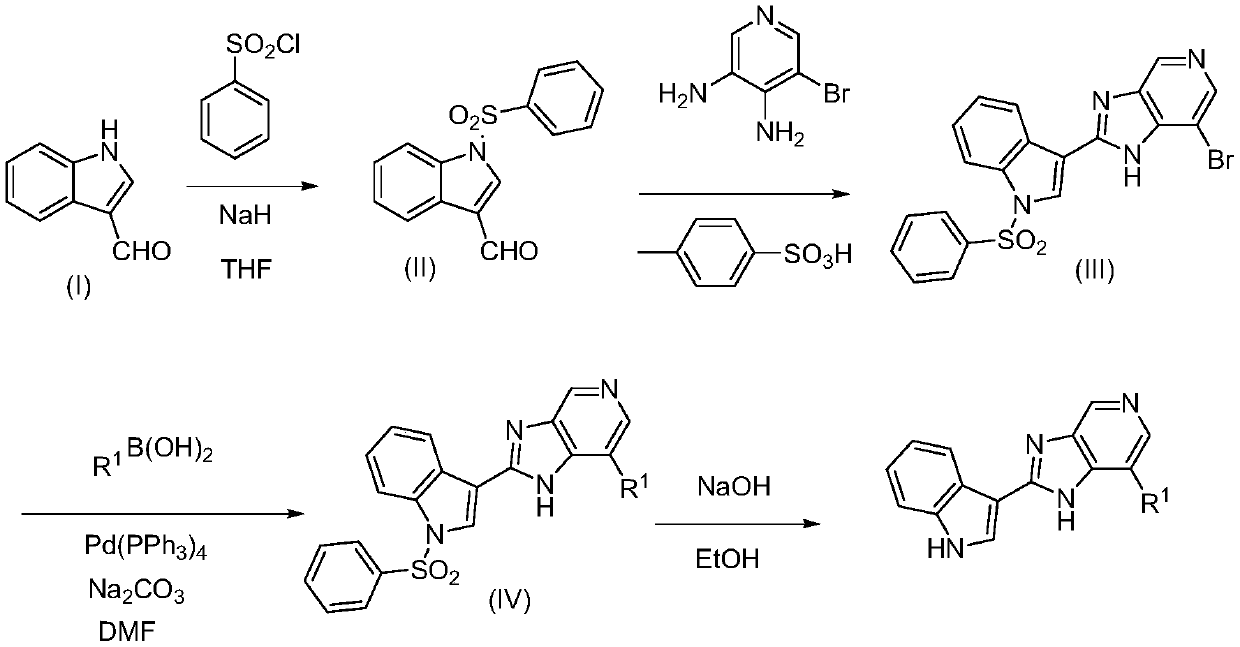
[0023] Embodiment 1 synthetic compound 1
[0024] Synthesis of Step 1 Compound II
[0025]
[0026] Dissolve 1.0 g of indole-3-carbaldehyde in 15 mL of tetrahydrofuran, then add 0.5 g of sodium hydrogen to the above solution, stir at room temperature for 5 h, then add 1.5 g of benzenesulfonyl chloride, and continue stirring for 8 h. TLC monitoring. After the reaction was completed, water was added to the reaction liquid to quench the reaction, and then extracted with ethyl acetate-water, the ethyl acetate layer was dried with anhydrous sodium sulfate, and the solution was concentrated to obtain 1.7 g of solid compound II. Yield 86.5%.
[0027] The obtained compound II is identified by nuclear magnetic resonance spectrum and mass spectrometry, and the identification results are:
[0028] 1 H NMR (CDCl 3 )δ10.13(s,1H),8.29(m,2H),8.01(m,3H),7.64(t,1H),7.54(t,2H),7.46-7.37(m,2H).MS(ESI )m / z Calcd.For[M+H] + :286.1; found: 286.1.
[0029] Synthesis of Step 2 Compound III...
Embodiment 2
[0043] Synthesis of compound 2
[0044] The synthesis method of compound 2 uses indole-3-carbaldehyde and 4-isoquinoline boronic acid as raw materials, and the method is the same as in Example 1.
[0045]
[0046] Compound 2 was prepared in the same manner as in Example 1 except that the corresponding raw materials were used, and the yield was 40.0%.
[0047] The obtained compound is identified by nuclear magnetic resonance spectrum and mass spectrometry, and the identification results are:
[0048] 1 H NMR (DMSO-d 6 )δ13.13(s,1H),11.80(s,1H),9.49(s,1H),9.03(br,1H),8.69(s,2H),8.31(s,3H),7.77(t,2H ),7.50(d,2H),7.21(br,2H).MS(ESI)m / z Calcd. For[M+H] + :362.1; found: 362.1.
Embodiment 3
[0050] Synthesis of compound 3
[0051] The synthesis method of compound 3 uses indole-3-carbaldehyde and (2-picoline 4-yl)boronic acid as raw materials, and the method is the same as that of Example 1.
[0052]
[0053] Compound 3 was prepared in the same manner as in Example 1 except that the corresponding raw materials were used, and the yield was 41.5%.
[0054] The obtained compound is identified by nuclear magnetic resonance spectrum and mass spectrometry, and the identification results are:
[0055] 1 H NMR (DMSO-d 6 )δ13.16(s,1H),11.88(s,1H),8.85(s,1H),8.75(s,1H),8.63(d,1H),8.58(m,1H),8.35(d,2H ),8.20(d,1H),7.57(s,1H),7.31-7.23(m,2H),2.64(s,3H).MS(ESI)m / z Calcd. For[M+H] + :326.1; found: 326.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com