Application of mycobacterium tuberculosis protein
A Mycobacterium tuberculosis protein technology, which is applied in the application field of Mycobacterium tuberculosis protein, can solve the problems of cross-contamination, good sensitivity, and clinical application deviation, and achieve high reliability results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
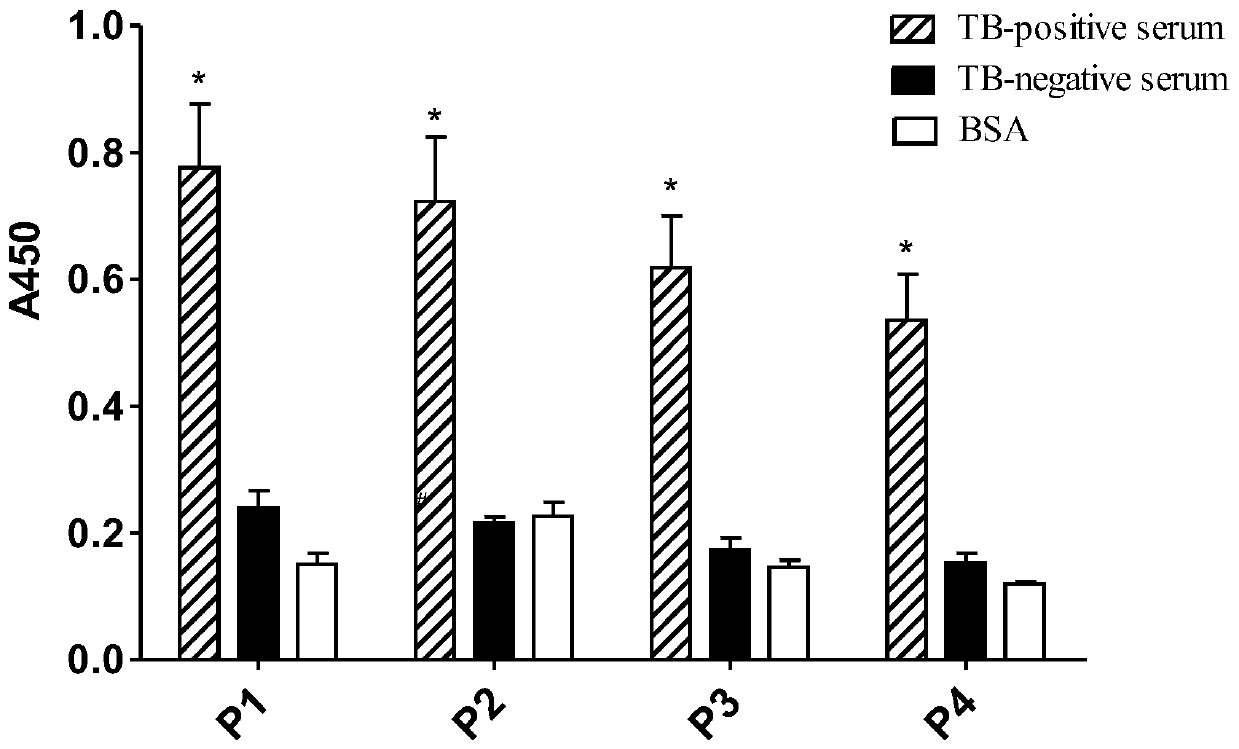
[0028] Example 1: ELISA detection test for specific binding of representative phages P1 to P4 to TB positive serum
[0029] Coat 100 μL of TB positive serum (100 μg / mL) on the microplate (set up negative serum control and BSA control at the same time), and incubate overnight at 4°C in a humid chamber. After discarding the coating solution, block with blocking solution (5% skimmed milk powder) for 2 h, wash with PBST 8 times, and add 150 μL of amplified representative phage (3×10 11 pfu, the T7 phage whose surface displays the protein encoding the sequence shown in SEQ ID NO:1-4), incubated at 37°C for 2h, washed 8 times with PBST, added 1:1000 diluted HRP-labeled anti-T7 phage antibody, and incubated at 37°C After 2 hours, wash with PBST 8 times, add A and B chromogenic solutions respectively, keep away from light for 15 minutes at 37°C, and measure the absorbance value at 450nm (A450) with a microplate reader after adding the stop solution.
[0030] Such as figure 1 Shown: ...
Embodiment 2
[0031] Example 2: Dot immunoblotting test for specific binding of representative phages P1 to P4 to TB positive sera
[0032] Wash the transparent PVDF membrane soaked in methanol with TBST (0.5% Tween 20), and add 1 μL of amplified representative phage (10 μL) dropwise to the membrane. 11 pfu, surface-displayed T7 phage encoding the sequence protein shown in SEQ ID NO: 1-4), after drying at room temperature, put it in a 4°C refrigerator to seal overnight, wash the membrane 3 times with TBST, and add the preliminary purified TB positive serum ( 1:50), incubated at 37°C for 2 h, washed the membrane 6 times with TBST, added HRP-labeled goat anti-human IgG antibody (1:4 000) and soaked the membrane at 37°C for 1 h, washed the membrane 6 times with TBST, and then emitted light by ECL method. Develop and fix. At the same time, negative control, blank control, wild-type phage control and positive control were set up.
[0033] In order to further confirm that representative phages ...
Embodiment 3
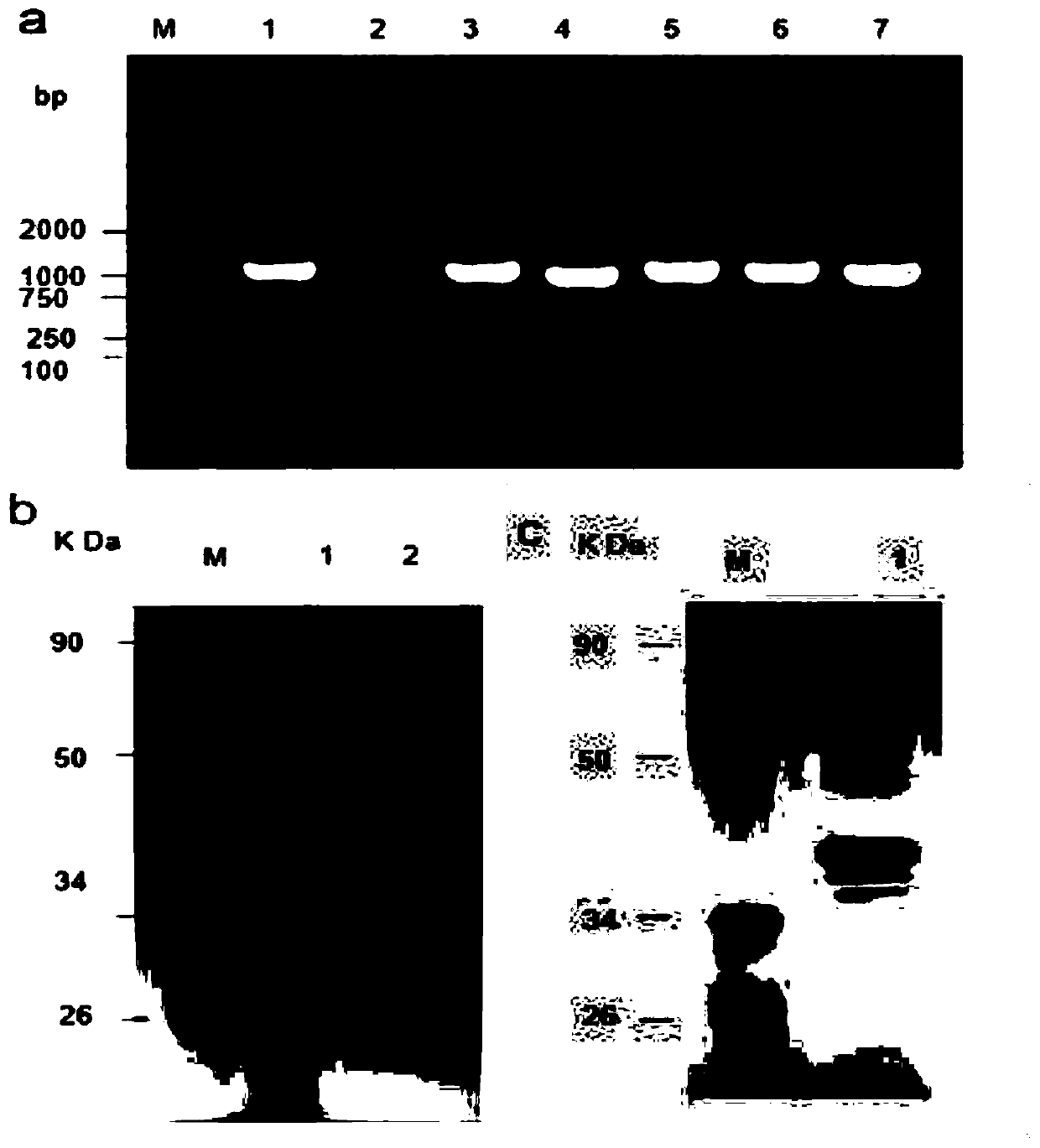
[0034] Embodiment 3: express and purify recombinant RK
[0035] A prokaryotic expression vector containing RK gene was constructed, and recombinant ribokinase was expressed and purified. The results showed that a PCR product with a size of 975 bp was successfully amplified, which had 100% homology with the DNA sequence of the RK gene. The prokaryotic expression vector pET-28a(+)-RK( image 3 a), expressed and purified the recombinant protein (36kDa) containing histidine tag ( image 3 b); if image 3 As shown in c, SDS-PAGE analysis shows that the purified recombinant protein appears as a single band. These results demonstrate the successful expression and purification of the recombinant ribokinase full-length protein. As identified by Western blot, the recombinant RK can specifically combine with the anti-histidine tag antibody and tuberculosis positive serum ( Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com