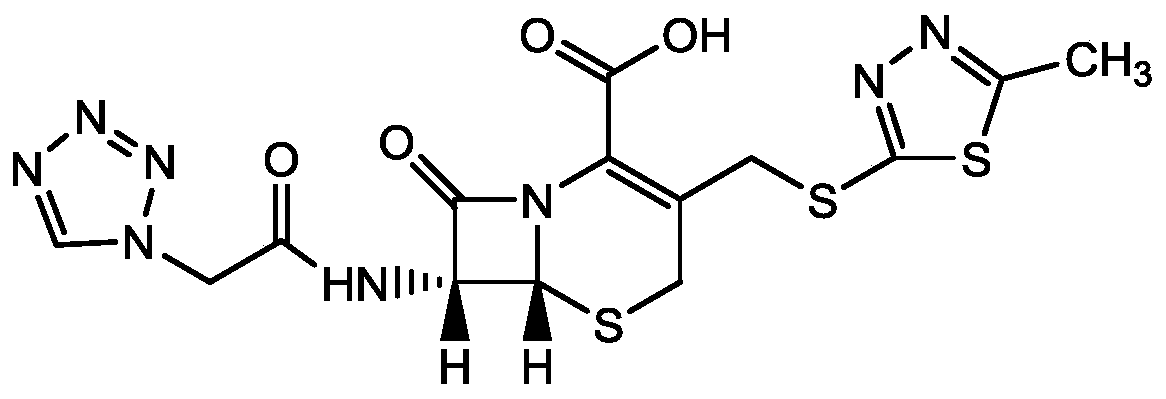
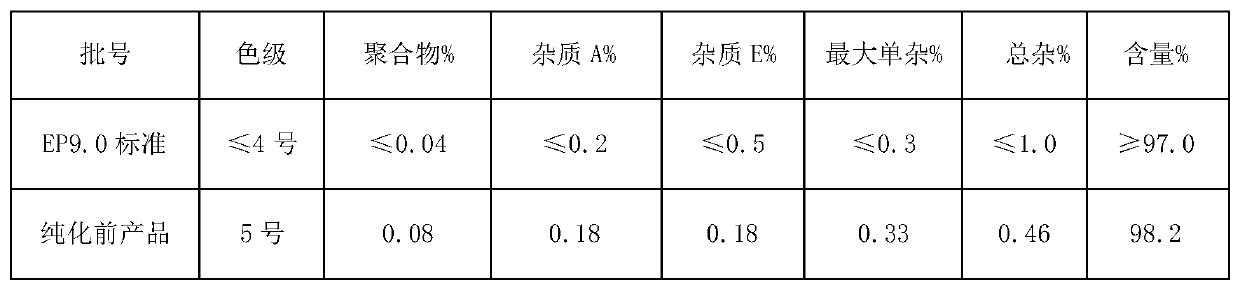
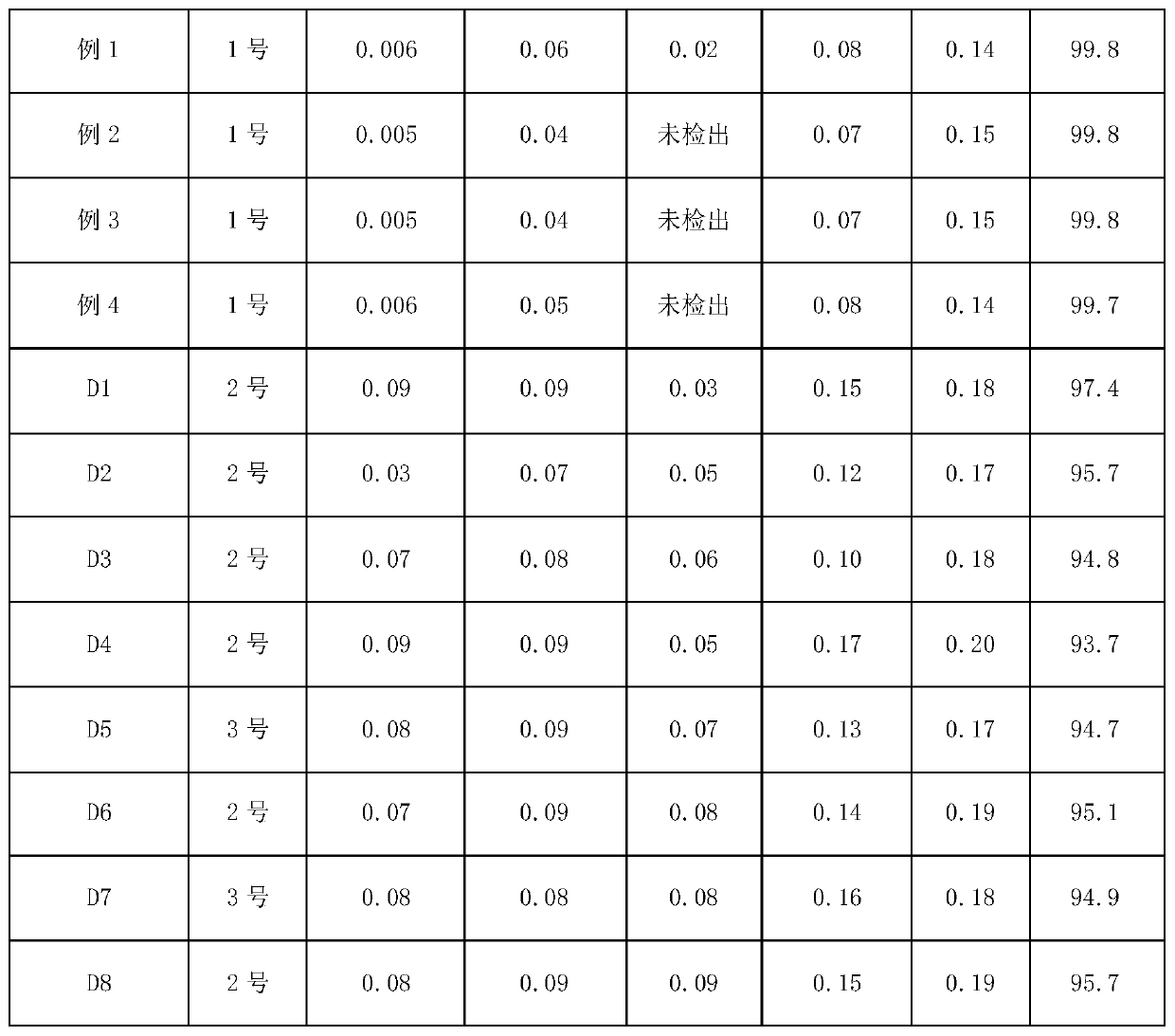
Purification method of cefazolin acid
A technology of cefazolinic acid and purification method, applied in the field of medicine, can solve the problems of unstable purification process, complex synthesis route, long process and the like, and achieve the effects of less visible foreign matter, high yield and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for purifying cefazolin, using cefazolin that does not meet quality requirements as raw material, comprising the steps of:
[0036] A. Take 20g of cefazolin acid, add 80ml of purified water, control the temperature at 20°C, add saturated sodium carbonate solution to adjust the pH of the system to 6.3, and stir until it dissolves;
[0037] B. Add 100mL ethyl acetate and stir for 10min, let stand for 10min to extract and separate phases, keep the water phase;
[0038] C. Add 5mL acetone and 5mL buffer solution to the water phase, control the temperature at 15°C, and add dropwise hydrochloric acid to adjust the pH of the system to 4.0, then add 0.1g of cefazolin acid seed crystals, and grow crystals for 30min at a time; wherein the buffer solution consists of It is prepared from citric acid and disodium hydrogen phosphate plus purified water. The mass volume of citric acid in the buffer solution is 11.8g / L, and the mass volume of disodium hydrogen phosphate is 27....
Embodiment 2
[0042] A method for purifying cefazolin, using cefazolin that does not meet quality requirements as raw material, comprising the steps of:
[0043] A. Take 20g of cefazolin acid, add 60mL of purified water, control the temperature at 25°C, add saturated sodium bicarbonate solution to adjust the pH of the system to 6.1, and stir until it dissolves;
[0044] B. Add 60mL of dichloromethane and stir for 10min, let it stand for 10min, extract and separate phases, and keep the water phase;
[0045] C. Add 10mL acetone and 5mL buffer solution to the water phase, control the temperature at 18°C, and add dropwise hydrochloric acid to adjust the pH of the system to 4.3, then add 0.02g cefazolin acid seed crystals, and grow crystals for 60min at a time; wherein the buffer solution consists of It is prepared from citric acid and disodium hydrogen phosphate plus purified water. The mass volume of citric acid in the buffer solution is 11.8g / L, and the mass volume of disodium hydrogen phosph...
Embodiment 3
[0049] A method for purifying cefazolin, using cefazolin that does not meet quality requirements as raw material, comprising the steps of:
[0050] A. Take 20g of cefazolin acid, add 70mL of purified water, control the temperature at 22°C, add saturated sodium carbonate solution to adjust the pH of the system to 6.2, and stir until it dissolves;
[0051] B. Add 80mL ethyl acetate and stir for 20min, let stand for 30min, extract and separate phases, keep the water phase;
[0052] C. Add 10mL acetone and 5mL buffer solution to the mixed solution, control the temperature at 17°C, and add dropwise hydrochloric acid to adjust the pH of the system to 4.18, then add 0.05g cefazolin acid seed crystals, and grow crystals for 45min at a time; wherein the buffer solution consists of It is prepared from citric acid and disodium hydrogen phosphate plus purified water. The mass volume ratio of citric acid in the buffer solution is 11.8g / L, and the mass volume ratio of disodium hydrogen phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com