Heterogeneous catalytic distillation process for preparing propionate by ester exchange
A heterogeneous catalysis and propionate technology, which is used in the preparation of mutual reaction between ester groups, chemical industry, sustainable manufacturing/processing, etc. , harsh temperature requirements, etc., to achieve the effect of short reaction time, short reaction path and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
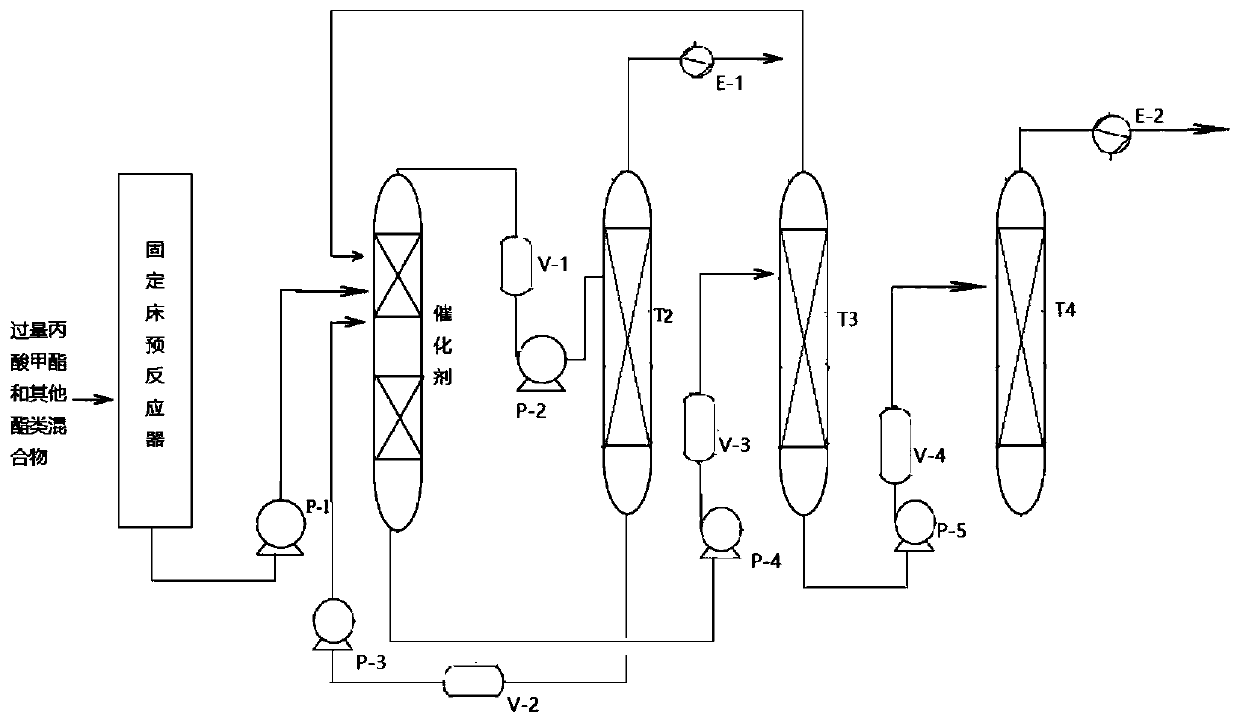
Image
Examples
Embodiment 1
[0041] Using methyl propionate and propyl acetate as raw materials, the ratio of propyl propionate to methyl acetate is 1:1, and the excess methyl propionate is recycled.
[0042] Catalyst 7%NiO-13%Co 2 o 3 The amount of / Li-Y is 0.3-5% of the total mass of the raw materials, and the ratio of the cross-sectional area of the initial distillation section to the side line section is 1:1.
[0043] The operating conditions are as follows:
[0044] Distillation tower T1: tower diameter 1000mm; tower height 19000mm; the number of plates in the public rectification section, initial distillation section, side line section, and public stripping section are 10, 20, 30, and 15 respectively; the pressure inside the tower is 1 MPa; The temperature is 60°C; the bottom temperature of the common stripping section is 71°C; the bottom temperature of the side line section is 74°C.
[0045] T2 rectification tower: tower diameter 3000mm; tower height 40000mm; the number of trays in the public ...
Embodiment 2
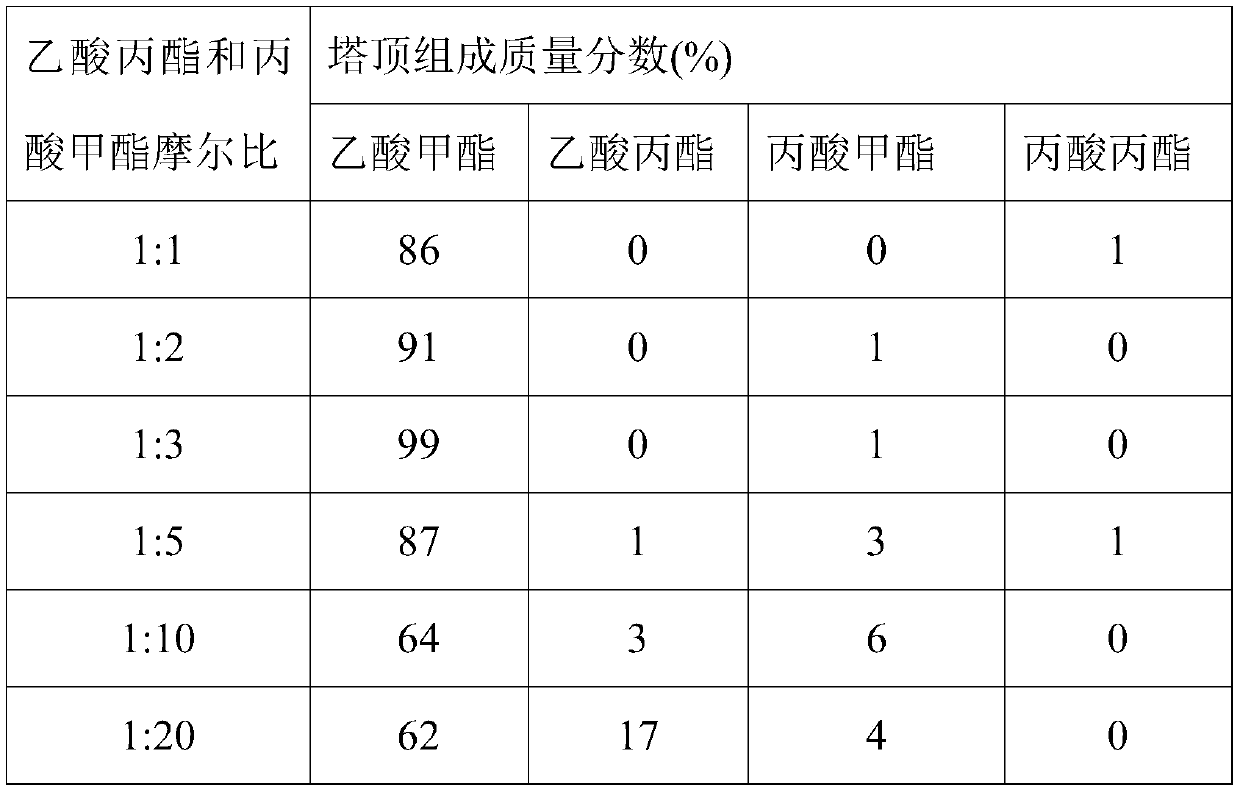
[0053] Under the operating conditions of Example 1, when the total mass flow rate of raw materials is 5000kg / h, changing the molar ratio of raw materials in the T1 rectification tower, the changes in the mass fractions of the components at the top of the tower are shown in Table 2.
[0054] Table 2 The influence of different molar ratios of raw materials on the mass fraction of each component at the top of the T1 distillation column
[0055]
[0056] As can be seen from Table 2, with the increase of the raw material molar ratio, the mass fraction of methyl acetate and methyl propionate at the top of the tower first increases and then decreases, and the mass fraction of propyl acetate increases gradually. When propyl acetate and propionic acid The best raw material ratio is when the molar ratio of methyl ester is 1:3.
Embodiment 3
[0058] Under the operating conditions of Example 2, the total raw material mass flow rate is 5000kg / h, and when the molar ratio of propyl acetate and methyl propionate is 1:3, the mass fraction of each component at the top of the tower changes when the reflux ratio of the T1 rectification column is changed. as shown in Table 3.
[0059] Table 3 Effects of different reflux ratios on the mass fraction of each component at the top of the T1 distillation column
[0060]
[0061]
[0062] As can be seen from Table 3, along with the increase of reflux ratio, the mass fraction of methyl acetate at the top of the tower tends to a steady state, and the content of methyl propionate, propyl propionate and propyl acetate decreases, and the increase of visible reflux ratio contributes to More light methyl acetate at the top of the tower returns to the tower to participate in the reaction again to increase the conversion rate of reactants, but when the reflux ratio is 2:1, the product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com