Application of polyketone compound in herbicides
A technology of polyketides and herbicides, applied in the direction of herbicides and algicides, applications, biocides, etc., can solve the problems of unreported phytotoxic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
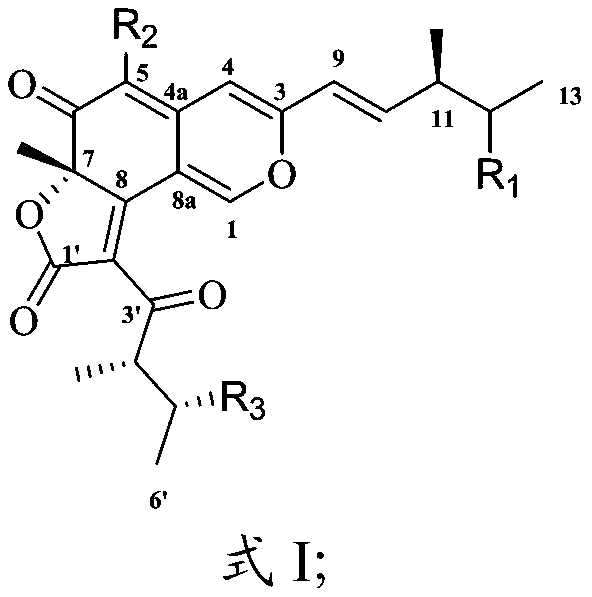
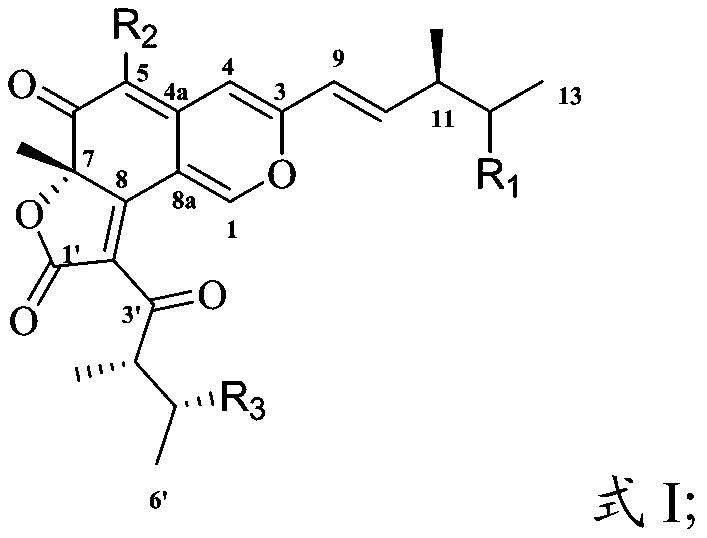
[0036] In the present invention, the preparation method of the compound having the structure shown in formula I preferably includes the following steps:
[0037] Cultivating endophytic fungi on a solid medium to obtain a fermentation product;
[0038] The fermentation product is mixed with an organic extraction solvent and then organically extracted to obtain a total extract;
[0039] The total extract is subjected to silica gel adsorption and elution in sequence, combined with thin layer analysis to obtain the corresponding subcomponents;
[0040] The corresponding subcomponents are sequentially subjected to chromatographic column separation and solvent evaporation to obtain a compound having the structure shown in formula I.
[0041] In the present invention, the plant endophytic fungus is preferably cultured on a solid medium to obtain a fermentation product.
[0042] In the present invention, the plant endophytic fungus is preferably Chaetomium cochliodes VTh01, Chaetomi...
Embodiment 1
[0091] Preparation of AL-9
[0092] Source of bacteria:
[0093] The fungus used is the plant endophyte Chaetomium globosum.
[0094] 1) fermentation
[0095] First prepare the solid medium for fungal fermentation. The specific formula is to add 60g of rice and 80mL of distilled water to a 500mL conical flask, and then autoclave at 121°C for 30 minutes for later use; For the amplification of strains, inoculate the amplified bacteria blocks on the rice medium for fermentation) and cut the fungi in the logarithmic phase into small pieces, inoculate them on the rice medium in the ultra-clean workbench for co-fermentation 50 bottles. Leave to ferment at room temperature for 15 days.
[0096] 2) Preparation of polyketide crude fraction from endophytic fungus Chaetomium globosum
[0097] After the fermentation of the above-mentioned strains is completed, obtain a solid fermentation product, soak the solid fermentation product with ethyl acetate, extract three times, reclaim the...
Embodiment 2
[0107] Preparation of AL-10
[0108] Dissolve the component Fr.E.I-D obtained in step 2) of Example 1 with chromatographic methanol, centrifuge, and pass through a 0.22 μm filter membrane for later use. The liquid chromatography conditions are 85% methanol to 15% water, and collect t R The chromatographic peak was 13.1 min. After the collection was completed, the preparation liquid was evaporated to dryness with a rotary evaporator to obtain monomer compound AL-10 (purity higher than 95%). After the samples were evaporated to dryness, some of the dried samples were taken out for NMR and mass spectrometry tests. The structure of compound AL-10 was identified by means of high-resolution mass spectrometry and one-dimensional and two-dimensional nuclear magnetic resonance.
[0109] AL-10: HR-ESI-MS (m / z433.1418[M+H] + , calcd.433.1418)
[0110] Nuclear magnetic resonance (1D and 2DNMR) further confirmed the compound structure: 1H-NMR (500MHz, CDCl 3 )δ8.70 (s, H-1), 6.62 (dd, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesh | aaaaa | aaaaa |
| Mesh | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com