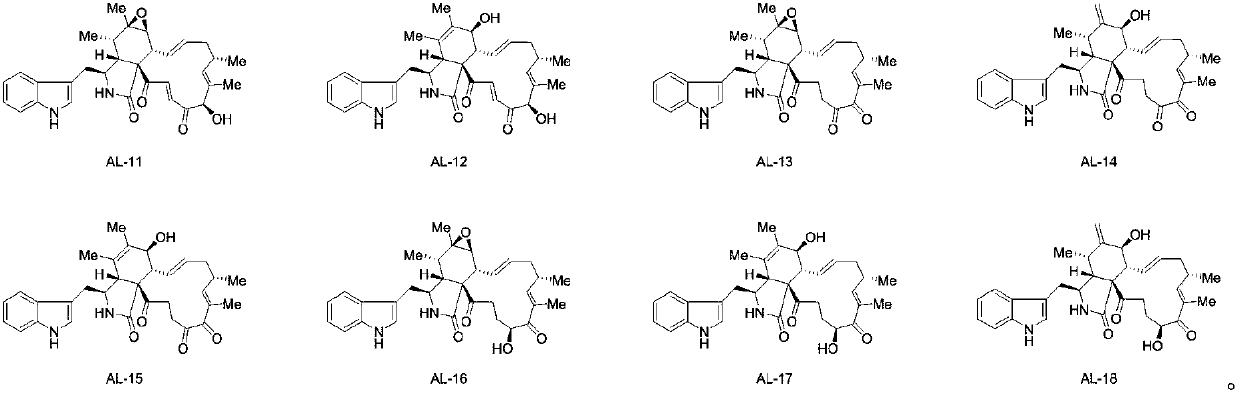
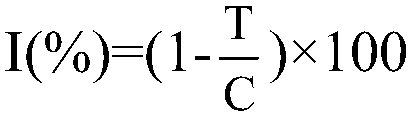Application of cytochalasin category compounds in herbicide
A cytochalasin and compound technology, applied in the field of pharmaceuticals, can solve problems such as unreported phytotoxic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the cytochalasin compounds is not particularly limited in the present invention, and can be obtained by conventional preparation methods known in the art. In the embodiment of the present invention, the preparation method of the cytochalasin compound preferably includes the following steps:
[0039] 1) Cultivating endophytic fungi on a solid medium to obtain a fermentation product;
[0040] 2) performing organic extraction after mixing the fermentation product obtained in step 1) with an organic extraction solvent to obtain the total extract;
[0041] 3) The total extract obtained in step 2) is sequentially subjected to silica gel adsorption and elution, combined with thin layer analysis to obtain subcomponents;
[0042] 4) The subcomponents obtained in step 3) are sequentially subjected to chromatographic column separation and solvent evaporation to obtain cytochalasin compounds.
[0043] In the invention, the plant endophytic fungus is cult...
Embodiment 1
[0094] Preparation of Cytochalasin Compounds
[0095] Source of bacteria:
[0096] The fungus used is the plant endophyte Chaetomium globosum.
[0097] 1) fermentation
[0098] First prepare the solid medium for fungal fermentation. The specific formula is to add 60g of rice and 80mL of distilled water to a 500mL conical flask, and then autoclave for 30 minutes under airtight conditions at 121°C for subsequent use; Base, used for the amplification of strains, inoculated the amplified bacteria block on the rice medium for fermentation) the fungus in the logarithmic phase was cut into small pieces, and inoculated into the rice culture in the ultra-clean workbench Basically, a total of 50 bottles were fermented and left to ferment at room temperature for 15 days;
[0099] 2) Preparation of crude fraction of cytochalasin from endophytic fungus Chaetomium gbosum
[0100]After the fermentation of the above-mentioned strains is completed, obtain a solid fermentation product, soak...
Embodiment 2
[0102] Preparation of compound AL-11
[0103] The component Fr-L obtained in step 2) of Example 1 was dissolved in chromatographic methanol, then centrifuged, and passed through a 0.22 μm filter membrane for use. Shimadzu chromatography and sunfire sequence chromatographic columns were used, and the mobile phase was 65% methanol to 35% water For chromatographic separation, collect t R After the chromatographic peak eluting at 39.6 min, after the collection, the preparation solution was evaporated to dryness with a rotary evaporator to obtain Fr-L-3. Dissolve the component Fr-L-3 obtained in the above steps with chromatographic methanol, then centrifuge, and pass through a 0.22 μm filter membrane for later use. Use green cotton chromatography, YMC chromatographic column, and the mobile phase is 65% methanol to 35% water for chromatography separate, collect R The liquid from the chromatographic peak at 22.6 min was collected and evaporated to dryness with a rotary evaporator t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com