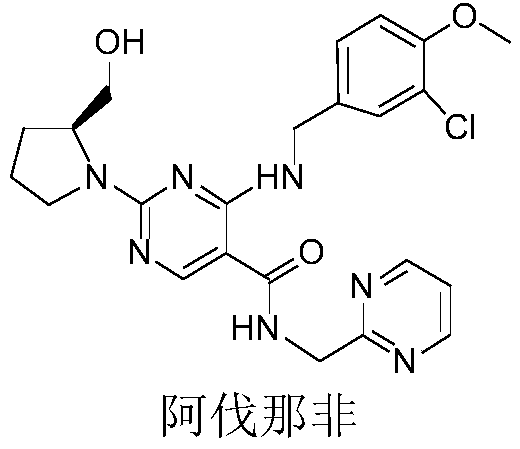
Method for detecting benzene and isopropylidene acetone residues in Avanafil by gas chromatography
A technology of mesityl oxide and gas chromatography, which is applied in the field of pharmaceutical analysis, can solve the problems of high toxicity and detection methods that cannot meet the detection sensitivity requirements, and achieve the effects of high separation, high sensitivity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Solution preparation:
[0035] Blank solvent: NMP-water (1:1) ratio mixing;
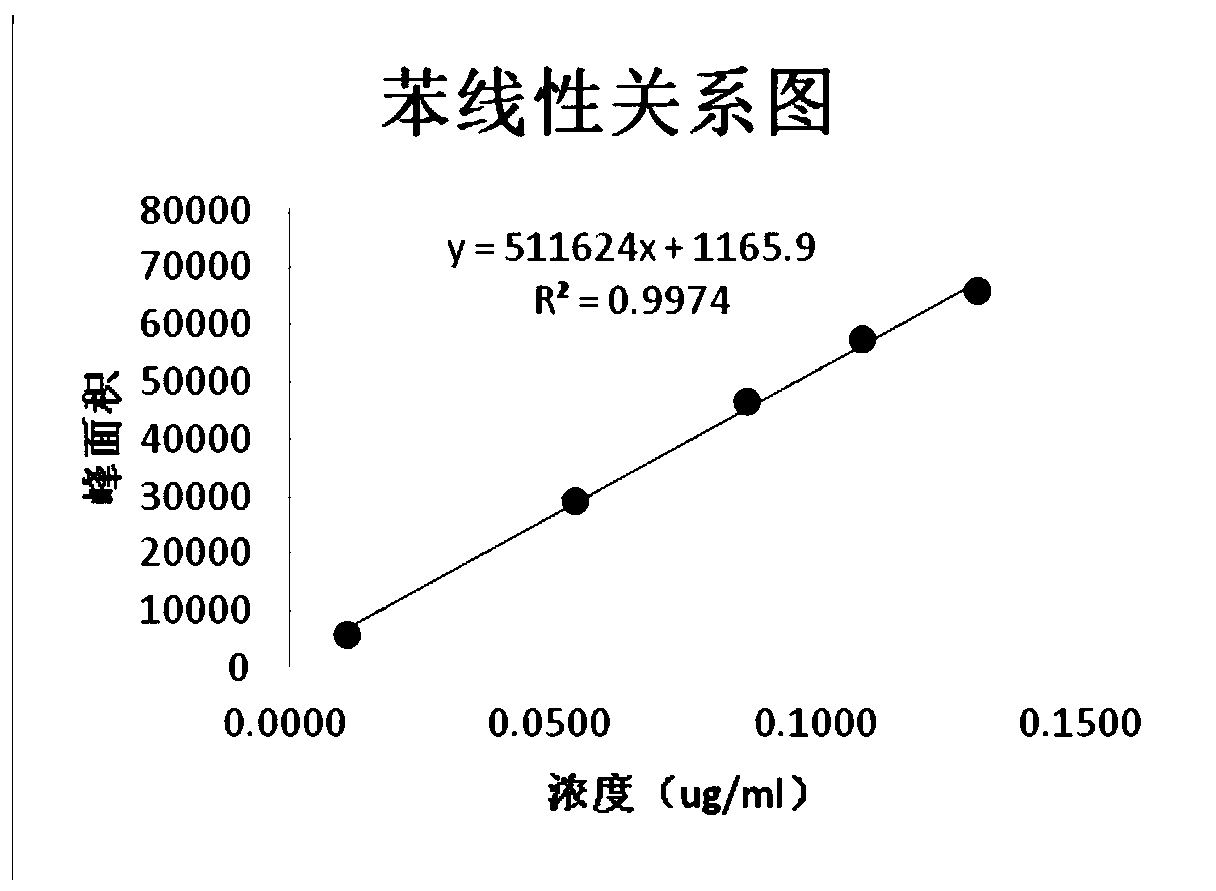
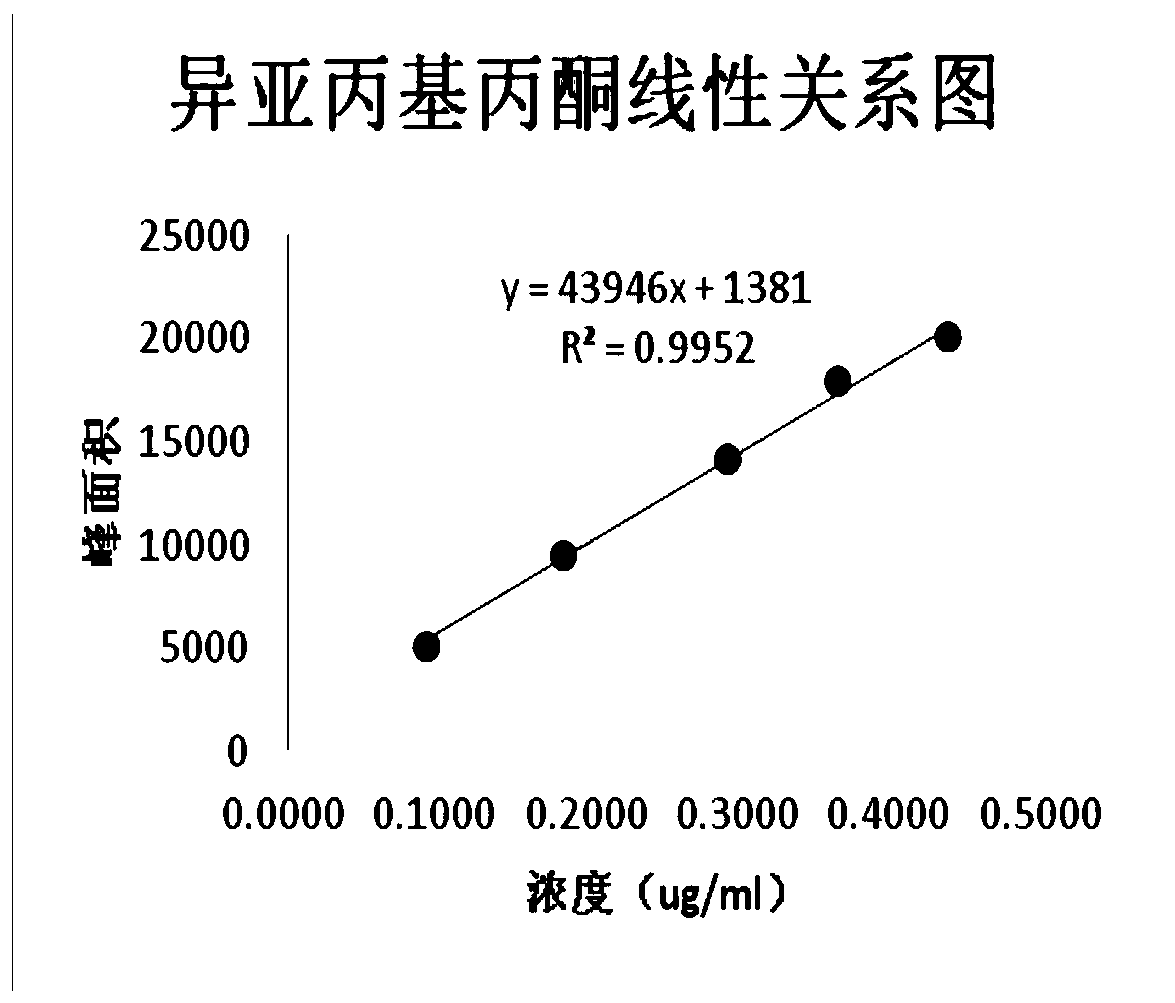
[0036] Reference solution: accurately weigh the appropriate amount of benzene and mesityl oxide, dissolve it with NMP-water (1:1) and dilute quantitatively to make a mixed control containing 0.1ug / ml of benzene and 0.375ug / ml of mesityl oxide The product solution, then accurately measure 5ml and place it in a 20ml headspace bottle, press the cap and seal, and get it;
[0037] Test solution: accurately weigh about 0.5g of the test product, place it in a 10ml measuring flask, add 5ml NMP precisely, shake it vigorously to dissolve it, then add water to dilute it to the mark and shake it well. Then precisely pipette 5ml into a 20ml headspace bottle, press the cap and seal, and it is ready;
[0038] (2) Gas chromatography detection:
[0039] The chromatographic column is Agilent DB-624 (30m*0.32mm*1.8um); the inlet temperature is 220℃; the detector temperature is 250℃; the detector is FID; the split ra...
Embodiment 2
[0045] Example 2 Method specificity test:
[0046] Accurately weigh about 0.5g of the sample to be tested, place it in a 10ml measuring flask, add 5ml of NMP precisely, shake it vigorously to dissolve it, add water to dilute it to the mark, and shake it well. Then precisely pipette 5ml into a 20ml headspace bottle, press the cap and seal, and then obtain the test solution. Accurately weigh an appropriate amount of benzene and mesityl oxide, dissolve it with NMP-water (1:1) and dilute quantitatively to make a mixed reference solution containing 0.1ug / ml of benzene and 0.375ug / ml of mesityl oxide. Accurately measure 5ml and place it in a 20ml headspace bottle, press the cap and seal to obtain the reference solution. Take blank solvent, reference solution, and test solution for headspace injection into the gas chromatograph. The results show that, under the chromatographic conditions of the present invention, the blank solvent does not interfere with the determination. The resolut...
Embodiment 3
[0047] Example 3 Method system suitability test
[0048] Take the reference solution of Example 2 for 5 consecutive injections, and the chromatographic data is shown in Table 1 below.
[0049] Table 1 Method system suitability test
[0050]
[0051] The results show that, under the conditions of the method of the present invention, the reference solution was injected 5 times continuously, and the RSD of the benzene peak area was 3.96%, which was less than 10%; the theoretical plate number was 159166, which was greater than 5000; The RSD is 2.20%, which is less than 10%, and the theoretical plate number is 473,617, which is greater than 5000, which proves that the method of the present invention has good system applicability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com