System and method for producing hydrogen peroxide through anthraquinone method
A hydrogen peroxide and anthraquinone method technology, which is applied in the anthraquinone method to produce hydrogen peroxide system and the anthraquinone method to produce hydrogen peroxide, can solve the problems of insufficient reaction, long gas-liquid movement path, waste of hydrogen resources, etc., and improve gas-liquid mass transfer. Effects of reduced rate, residence time and by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
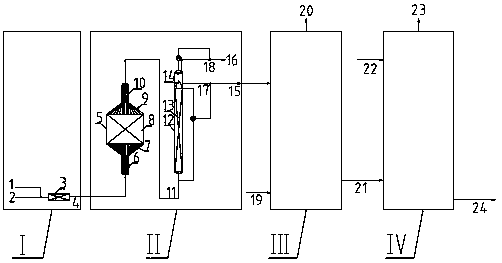
[0050] Using the present invention figure 1 The system in the production method of anthraquinone method. In the hydrogenation unit, the hydrogenation reactor is a fixed bed reaction and a tubular reactor in series, and the fixed bed reactor is filled with a catalyst of 0.15m 3 , The feeding section is filled with φ13, φ6 and φ3 inert ceramic balls in sequence, and the discharge section is filled with φ3, φ6 and φ13 inert ceramic balls in sequence. The upper and lower parts of the tubular reactor are filled with φ3 inert ceramic balls and hydrogenation catalysts in a volume ratio of 1:1. First, the working fluid 1.87m 3 / h with hydrogen 16.78Nm 3 / h mixed and then introduced into the top of the fixed bed reactor, and passed through the two beds of the fixed bed reactor sequentially from top to bottom. The reaction inlet temperature of the fixed bed reactor is 42-45°C, the reaction pressure is 0.32-0.38MPaG, and the reaction effluent enters the oxidation tower; the oxidation...
Embodiment 2
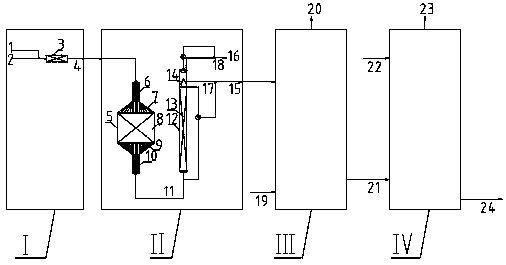
[0052] Using the present invention figure 2 The system in the production method of anthraquinone method. Among them, the hydrogenation reactor is a fixed bed reaction and a tubular reactor connected in series, and the inside of the fixed bed reactor is filled with a catalyst of 0.15m 3 , Both the feed section and the discharge section are filled with random saddle ring packing. The upper and lower parts of the tubular reactor are filled with saddle ring packing and hydrogenation catalyst in a volume ratio of 2:1. First, the working fluid 1.90m 3 / h with hydrogen 17.18Nm 3 / h mixed and then introduced into the bottom of the fixed-bed reactor, and passed through the two beds of the fixed-bed reactor sequentially from bottom to top. The reaction inlet temperature of the fixed-bed reactor is 42-45°C, the reaction pressure is 0.32-0.38MPaG, and the reaction effluent oxidation tower; the oxidation tower is a packed tower structure, and the 1.88m 3 / h hydrogenated liquid and 77...
Embodiment 3
[0054] Using the present invention figure 2 The system in the production method of anthraquinone method. Among them, the hydrogenation reactor is a fixed bed reaction and a tubular reactor connected in series, and the inside of the fixed bed reactor is filled with a catalyst of 0.150m 3 , Both the feed section and the discharge section are filled with random saddle ring packing. The upper and lower parts of the tubular reactor are filled with saddle ring packing and hydrogenation catalyst in a volume ratio of 2:1. First, the working fluid 1.90m 3 / h with hydrogen 17.18Nm 3 / h mixed and then introduced into the bottom of the fixed-bed reactor, and passed through the two beds of the fixed-bed reactor sequentially from bottom to top. The reaction inlet temperature of the fixed-bed reactor is 42-45°C, the reaction pressure is 0.32-0.38MPaG, and the reaction effluent enters the oxidation tower; the oxidation tower is a packed tower structure, and the 1.90m 3 / h hydrogenated l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com