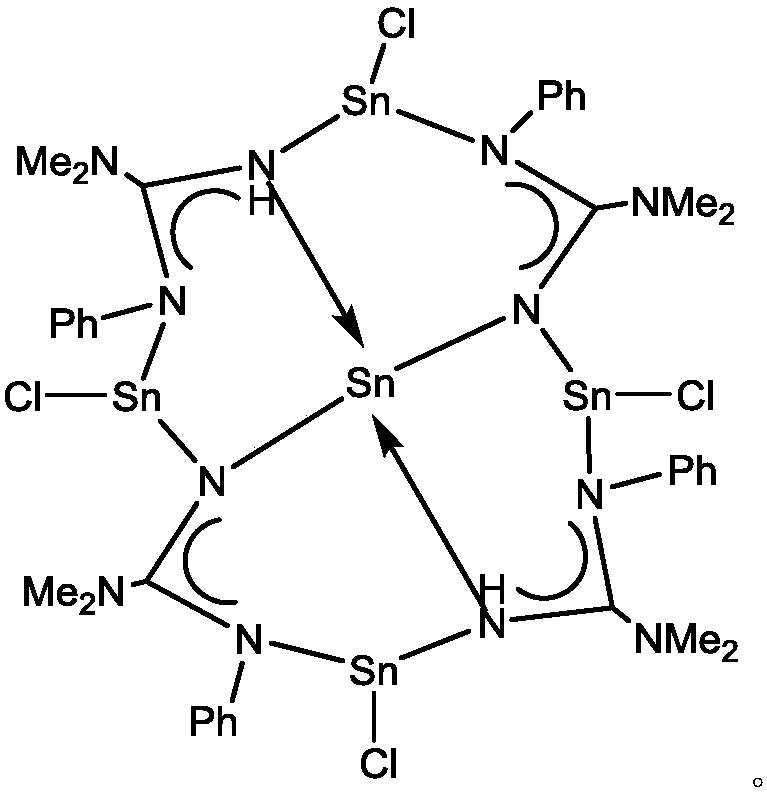
Five-core tin (II) compound and preparation method and application thereof
A compound, the technology of trimethylsilylbenzene, applied in the field of pentanuclear tin compounds and its preparation, can solve the problems of high cost of rare earth metals and complicated synthesis process, and achieve low synthesis cost, high sample purity and novel compound structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation of pentanuclear tin (II) compound a: Add 0.50 g (2.92 mmol) lithium trimethylsilylphenyl (base) amide to a weighed reaction flask under nitrogen protection, add 30 ml of ether, and place in an acetone bath Dimethylaminonitrile (0.24ml, 2.92mmol) was added under the conditions, reacted overnight, and SnCl was added under the conditions of the acetone bath. 2 (0.69g, 3.65mmol), slowly warmed up to room temperature, and reacted overnight. Stand still, filter, the filtrate is concentrated under vacuum, and crystallize at room temperature, and a colorless crystal is precipitated to obtain 0.256 g of pentanuclear tin (II) compound a, with a yield of 24.4%.
[0014] Melting point: 138-141°C
[0015] Elemental analysis: theoretical value C, 33.04; H, 3.74; N, 11.56%; found value C, 33.01; H, 3.72; N, 11.65.
[0016] NMR: 1 H NMR (CDCl 3 ):δ1.69(THF),2.57(s,12H,N(CH 3 ) 2 ),3.21(s,12H,N(CH 3 ) 2 ), 3.76(THF), 4.10(s,2H,NH),7.02-7.38(m,20H,Ph). 13 C NMR (CDCl...
Embodiment 2
[0018] Step 1, the preparation of pentanuclear tin (II) compound a is the same as in Example 1.
[0019] Step 2, the addition reaction of aniline and N,N'-diisopropylcarbodiimide: Weigh 0.015g (0.01mmol) of compound a into a 30mL Schlenk bottle filled with nitrogen, add N,N' - Diisopropylcarbodiimide 0.31 mL (2.00 mmol) and aniline 0.18 mL (2.00 mmol). Reacted at 25°C for 1.0h, hydrolyzed with 0.5mL of water, extracted three times with 30mL of dichloromethane (3×10mL), anhydrous Na 2 SO 4 Dry and filter. The filtrate was sucked dry, and the product was crystallized and purified with n-hexane to obtain the product guanidine with a yield of 80.4%.
Embodiment 3
[0021] Step 1, the preparation of pentanuclear tin (II) compound a is the same as in Example 1.
[0022] Step 2, the addition reaction of aniline and N,N'-diisopropylcarbodiimide: Weigh 0.015g (0.01mmol) of compound a into a 30mL Schlenk bottle filled with nitrogen, add N,N' - Diisopropylcarbodiimide 0.31 mL (2.00 mmol) and aniline 0.18 mL (2.00 mmol). Reacted at 25°C for 2.0h, hydrolyzed with 0.5mL of water, extracted three times with 30mL of dichloromethane (3×10mL), anhydrous Na 2 SO 4 Dry and filter. The filtrate was pumped dry, and the product was crystallized and purified with n-hexane to obtain the product guanidine with a yield of 84.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com