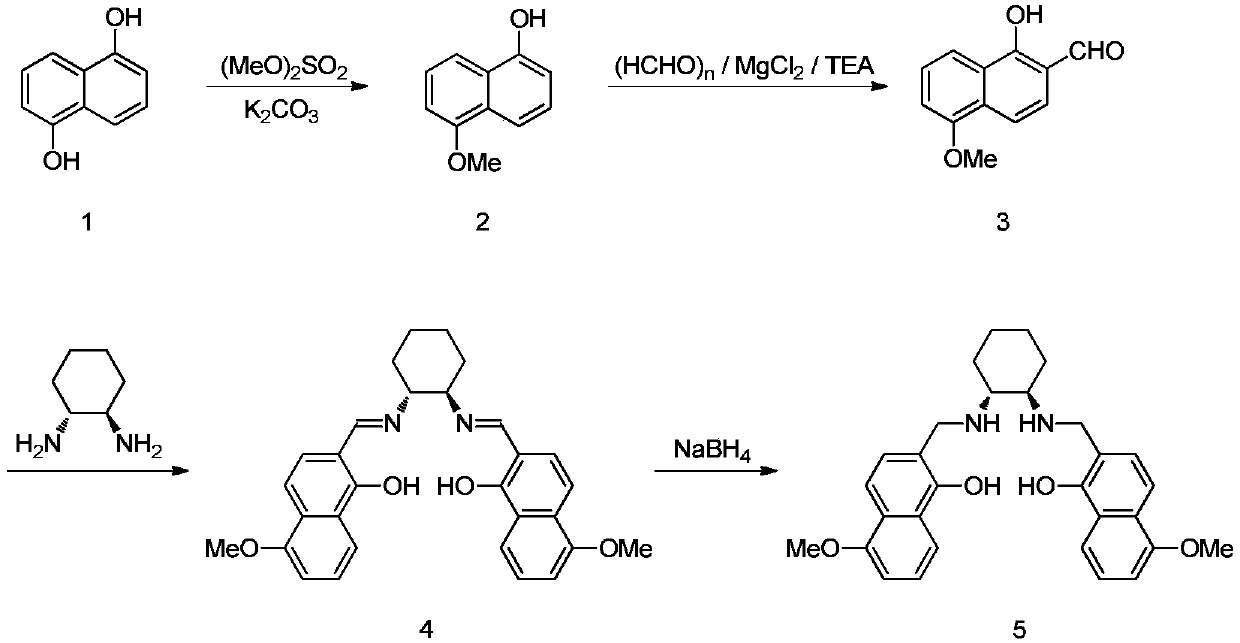
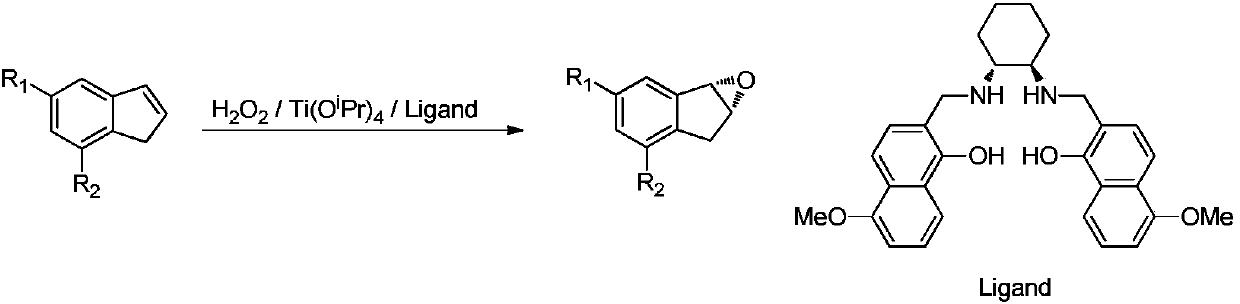
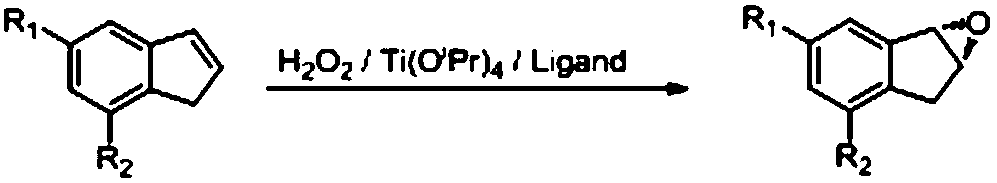Indene compound asymmetric epoxidation reaction ligand and preparation and application methods thereof
An epoxidation reaction and compound technology, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., can solve the problems of high cost, difficult preparation, complex structure, etc., and achieve simple operation, mild conditions, Effect of simple ligand structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] For the synthesis of the ligand, the substituent is methyl as a specific example below. Adoption of other substituents does not affect the technical effect of the present invention.
[0027]
[0028] Under nitrogen protection, add 160.2g of compound 1 to a 2L reaction flask, add 1L of acetone, start stirring, add 165.6g of potassium carbonate, cool in an ice-water bath to below 15°C, and dropwise add 151.3g of dimethyl sulfate , the exotherm is obvious, the temperature is controlled below 25°C, and stirred overnight at 28°C. Poured into 3L of ice water and stirred for 0.5 hours. Suction filtration, wash the filter cake (0.5L × 3). The filter cake was dissolved in 2L of MTBE and washed with saturated sodium chloride (1L). 50g of anhydrous sodium sulfate was dried and filtered. Concentrate under reduced pressure at 40°C to the remaining 1 / 3 solvent. Heat up to reflux, add 0.5 L of n-heptane, cool and crystallize. The product was collected by filtration and vacuum...
Embodiment 2
[0033] asymmetric epoxidation reaction
[0034]
[0035]Under the protection of nitrogen, add 20mL of dichloromethane in the reaction bottle of 250mL, add the ligand of 2.9g, start stirring. After adding 1.4 g of tetraisopropyl titanate, the system turned into a yellow solution, which was stirred at 25°C for 0.5 hours. 0.4 g of water was added; stirring was continued for 0.5 hours. 11.6 g of indene was added and 96 mL of dichloromethane was added. The temperature of the external bath was raised to 40°C, and 22.7g of 30% hydrogen peroxide was added dropwise, and the inner temperature was 38°C. After adding 40°C to react for 8 hours, separate the layers, extract the aqueous phase with dichloromethane (58mL), combine the organic phases, wash with 10% sodium thiosulfate (58mL), wash with water (58mL), and wash with 10g of anhydrous sodium sulfate dry. Concentrate under reduced pressure at 40°C and distill under reduced pressure to obtain 11.0 g with a yield of 83%, a purity...
Embodiment 3
[0037] Under nitrogen protection, add 400mL of dichloromethane to the 5L reaction flask, add 58g of ligand, and start stirring. After adding 28g of tetraisopropyl titanate, the system turned into a yellow solution, which was stirred at 25°C for 0.5 hours. 8 g of water were added; stirring was continued for 0.5 hours. 232 g of indene was added and 1920 mL of dichloromethane was added. The temperature of the external bath was raised to 40°C, and 454g of 30% concentration of hydrogen peroxide was added dropwise, and the inner temperature was 38°C. After adding 40°C to react for 12 hours, separate the layers, extract the aqueous phase with dichloromethane (1160 mL), combine the organic phases, wash with 10% sodium thiosulfate (1160 mL), wash with water (1160 mL), and wash with 150 g of anhydrous sodium sulfate dry. Concentrate under reduced pressure at 40°C, and distill under reduced pressure to obtain 225 g with a yield of 85%, a purity of 98.2%, and an ee of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com