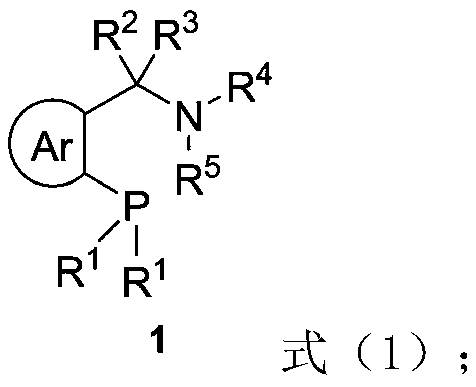
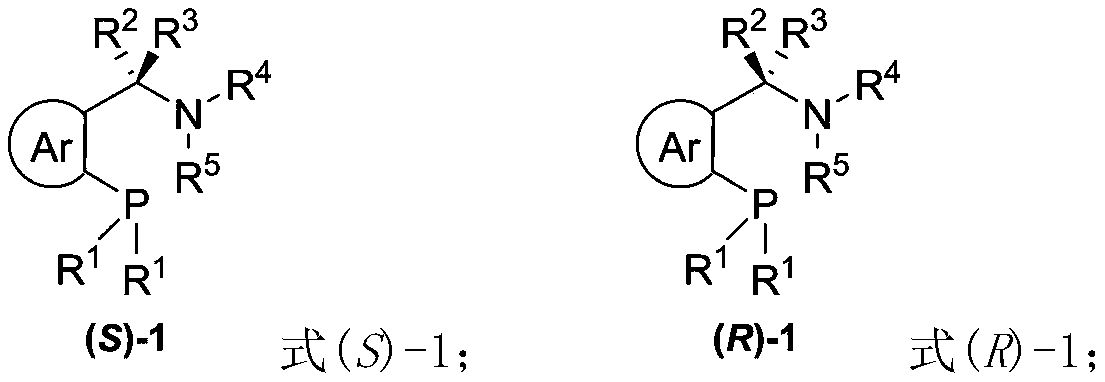
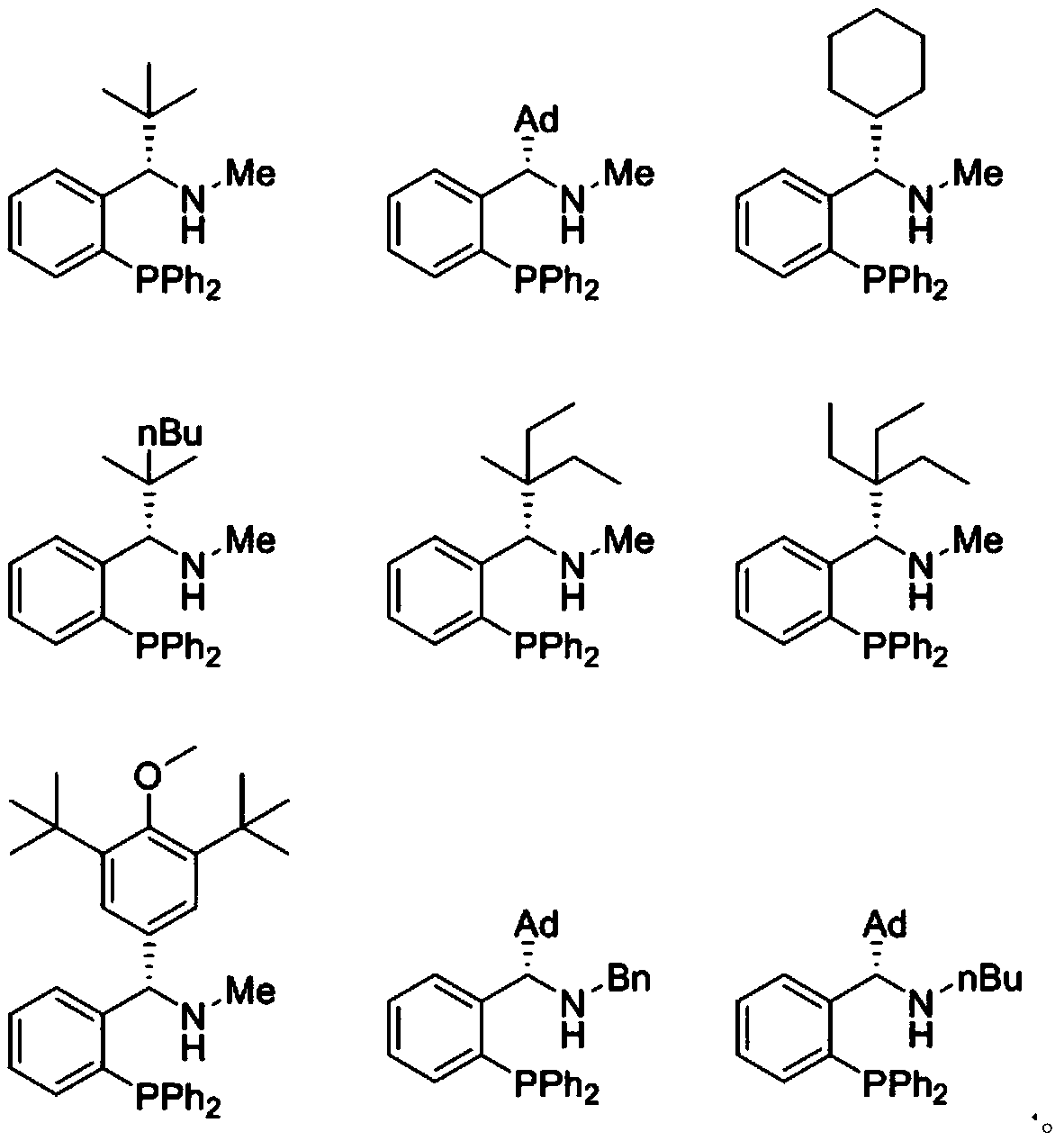
Chiral bidentate nitrogen phosphine ligand Rong-Phos as well as preparation method and application thereof
A bidentate nitrogen and phosphine ligand technology, applied in organic chemistry methods, chemical instruments and methods, catalytic reactions, etc., can solve the problem that the chirality of the nitrogen atom is easy to flip and unstable, the influence and effect of the nitrogen atom center have not been studied, and the chiral Problems such as sexuality are not considered by people, and achieve the effect of many modifiable sites, excellent reactivity and high enantioselectivity, and simple ligand structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 (S C , R S )-6a synthesis
[0100] Refer to method 1, in a 50mL dry single-mouth bottle, add under helium atmosphere (1mmol) and dry tetrahydrofuran solvent (10mL), stirred at -40°C for 10 minutes, and added n-butyllithium (1.6eq., 1mL, 1.6M) dropwise. Stirring was continued for 1 hour, methyl triflate (1.6 mmol) was added, followed by stirring for 30 minutes. The reaction was then quenched with saturated ammonium chloride solution. Separation, extraction, drying, spin-drying solvent and column chromatography purification were carried out again to obtain The yield was 91%. Proton NMR (500MHz, Chloroform-d) δ7.76–7.69(m,1H),7.37–7.21(m,11H),7.18–7.11(m,1H),7.11–7.04(m,1H),5.43( d, J=13.6Hz, 1H), 2.62(s, 3H), 1.13(s, 9H), 1.01(s, 9H). Phosphorus NMR (202MHz, CDCl 3 )δ-16.37. Carbon NMR (126MHz, Chloroform-d) δ145.99(d, J=24.4Hz), 137.31(d, J=10.6Hz), 137.08(d, J=2.7Hz), 136.99(d ,J=3.4Hz),135.39,133.92(d,J=6.9Hz),133.76(d,J=7.1Hz),129.57(d,J=3.6Hz),1...
Embodiment 2
[0101] Example 2 (S C , R S )-6b synthesis
[0102] Concrete operation with reference to embodiment 1, used raw material is The yield was 86%. Proton NMR (500MHz, Chloroform-d) δ7.76–7.71(m,1H),7.43–7.39(m,1H),7.35–7.29(m,9H),7.28–7.23(m,3H),5.47( d, J=13.1Hz, 1H), 2.84(s, 3H), 1.88–1.73(m, 6H), 1.63–1.48(m, 9H), 1.08(s, 9H). Phosphorus NMR (202MHz, CDCl 3 )δ-18.14, carbon NMR (126MHz, Chloroform-d) δ143.89(d, J=25.9Hz), 137.80(d, J=11.9Hz), 137.33(d, J=12.3Hz), 137.15(d ,J=10.8Hz),136.16(d,J=1.8Hz),134.10(d,J=20.0Hz),133.45(d,J=19.0Hz),129.98(d,J=4.1Hz),128.67,128.51 –128.37(m), 128.33, 127.14, 73.65(d, J=27.5Hz), 58.66, 40.68(d, J=1.6Hz), 40.04, 30.10(d, J=1.4Hz), 28.62, 24.75(d, J=2.0Hz). High resolution mass spectrometry theoretical data C 34 h 43 NOPS([M+H] + ), 544.2797, experimental data 544.2794.
Embodiment 3
[0103] Example 3 (S C , R S )-6c synthesis
[0104] Concrete operation with reference to embodiment 1, used raw material is The yield was 76%. Proton NMR (500MHz, Chloroform-d) δ7.52–7.12(m,14H),5.23(t,J=10.2Hz,1H),2.64(s,3H),2.15–2.03(m,1H),2.00 –1.88(m,1H),1.84–1.75(m,1H),1.61–1.50(m,1H),1.40–1.27(m,2H),1.22–0.91(m,13H),0.85–0.78(m, 1H). Phosphorus NMR (202MHz, CDCl 3 )δ-18.64. Carbon NMR (126MHz, Chloroform-d) δ145.32(d, J=25.4Hz), 137.04(d, J=12.1Hz), 136.80–136.51(m), 135.02, 134.53(d, J=20.7Hz), 133.57(d, J=18.8Hz), 129.16, 128.89, 128.66–128.32(m), 127.15, 126.86(d, J=4.9Hz), 70.10(d, J=26.3Hz), 58.61 C 30 h 38 NNaOPS([M+Na] + ), 514.2304, experimental data 514.2297.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com