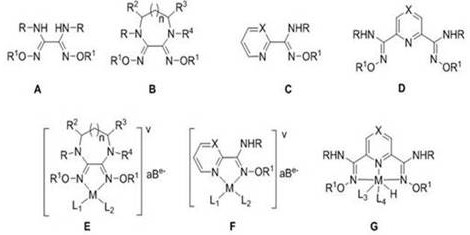
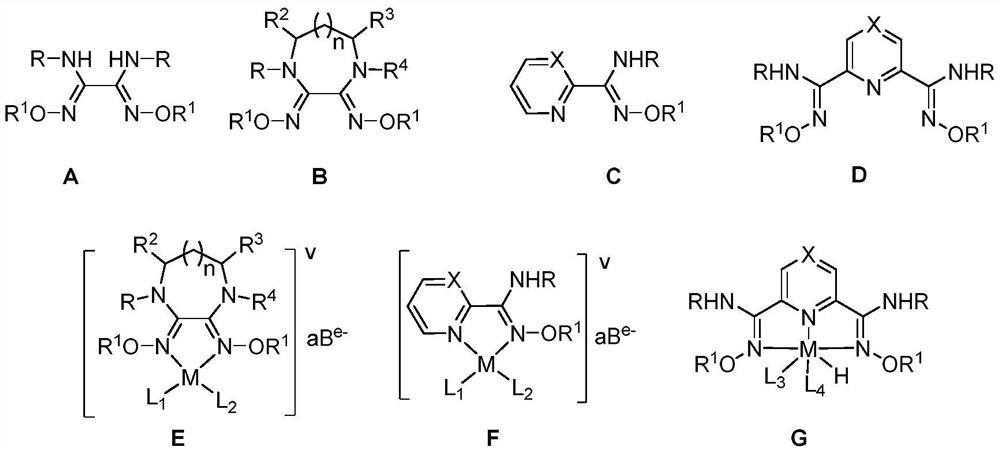
A Class of Formic Acid Dehydrogenation Catalyst and Its Application
A technology of catalyst and catalyst concentration, applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, hydrogen, etc. Low efficiency and other problems, to achieve the effect of high catalytic efficiency, simple structure and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
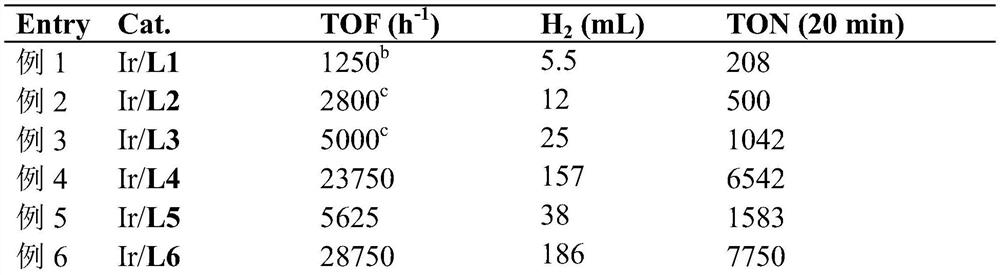
[0025] Use glyoxaldoxime L1 as ligand, FA (1.0M, 10.0mL), 60°C.
[0026] Weigh [Cp*IrCl 2 ] 2 (4.0mg, 5.0μmol) and L1 (1.42mg, 12μmol) were placed in a reagent bottle, and 1.0mL of purified water was added to prepare an aqueous solution of the in-situ catalyst. Add magnetons, water (9.42mL) and formic acid (10mmol, 0.38mL) to a Schlenk reaction tube or bottle, seal with a rubber stopper, connect the branch to the rubber tube; put the reaction bottle into a water bath at 60°C and stir to stabilize After 10 minutes, pipette 0.2mL (1.0μmol) of the prepared catalyst solution into the reaction bottle quickly with a pipette gun, seal the reaction bottle, and immediately put the rubber hose connected to the branch pipe into the water basin, fill it with water and stand it upside down In a 500mL graduated cylinder, the timer starts, and the gas is collected by the drainage method. Calculate the amount of gas collected per unit time, calculate TON and TOF, V(CO 2 )=Volume / 2 of the ...
Embodiment 2
[0028] Same as Example 1, except that the ligand (Z)-N'-hydroxypicolinimidamide L2 was used instead of L1 for the reaction, and the results are shown in Example 2 in Table 1.
Embodiment 3
[0030] The same as in Example 1, except that the ligand 1,2-dimethyldimethylacetate oxime L3 was used instead of L1 for the reaction, and the results are shown in Example 3 in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com