Free nucleic acid preservative and blood collection and preservation device
A free nucleic acid and preservative technology, applied in biochemical cleaning devices, enzymology/microbiology devices, biological material sampling methods, etc. and other problems, to prevent DNA dilution and contamination of target cfDNA, improve shelf life, and slow down the effect of rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 reagent
[0034] According to the different anticoagulants used, the formulations were divided into three groups. For the sake of simplification, the following experimental formulations all used glutamine 10mg / ml, sodium orthovanadate 800mg / ml, leupeptin 5mg / ml, dye Genistein 5mg / ml, Aluminum Fluoride 1mg / ml, Acetylcysteine 40mg / ml, Diazolidinyl Urea 100mg / ml. The table below shows only the representative ingredient ratios used in the final summary verification, and some wrong or unrepresentative ratios in the previous period are not shown.
[0035] During the preparation, dissolve the required amount of various reagents in RNase-free purified water.
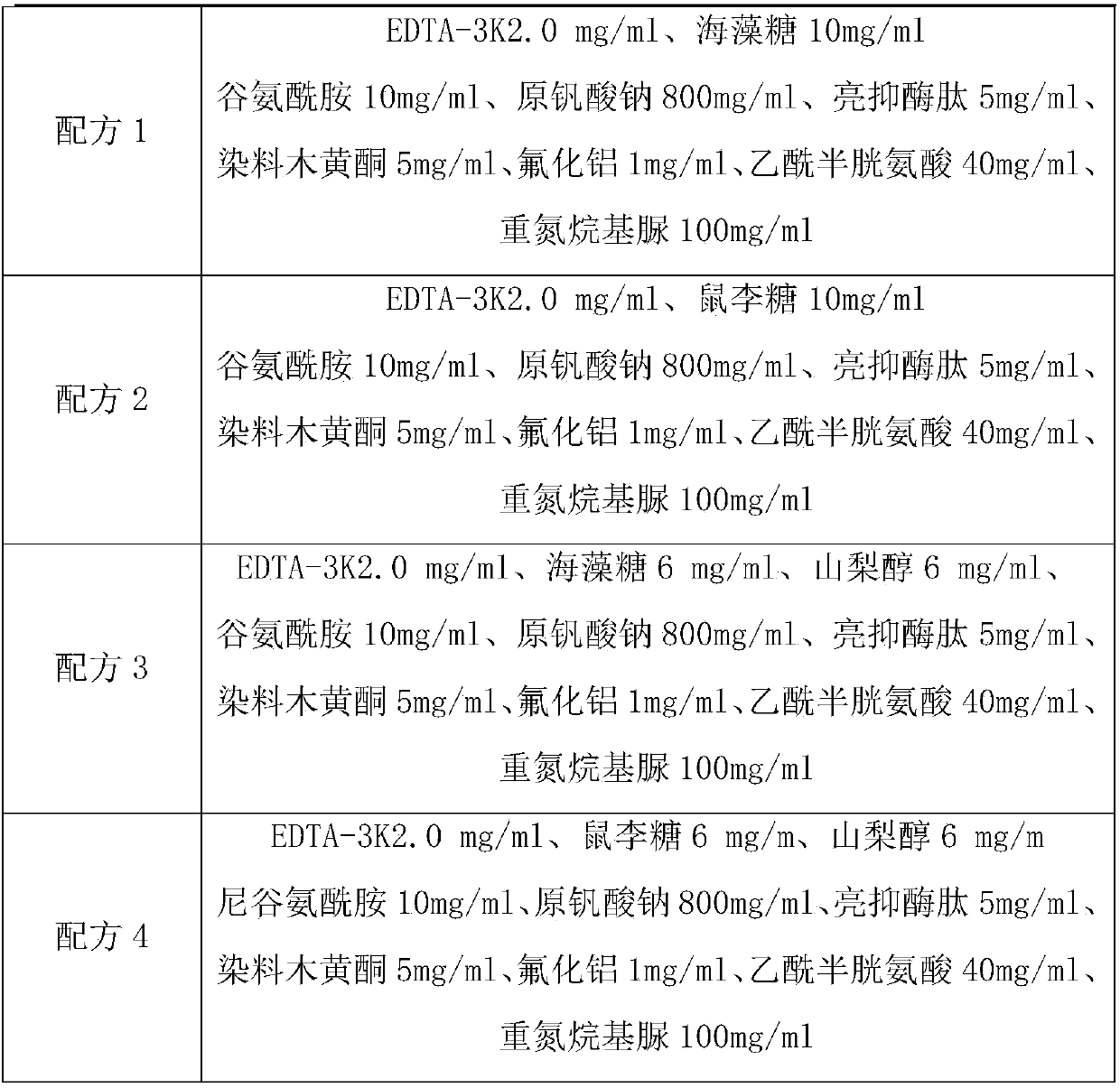
[0036] Table 1 Formula based on EDTA-3K anticoagulant
[0037]
[0038]
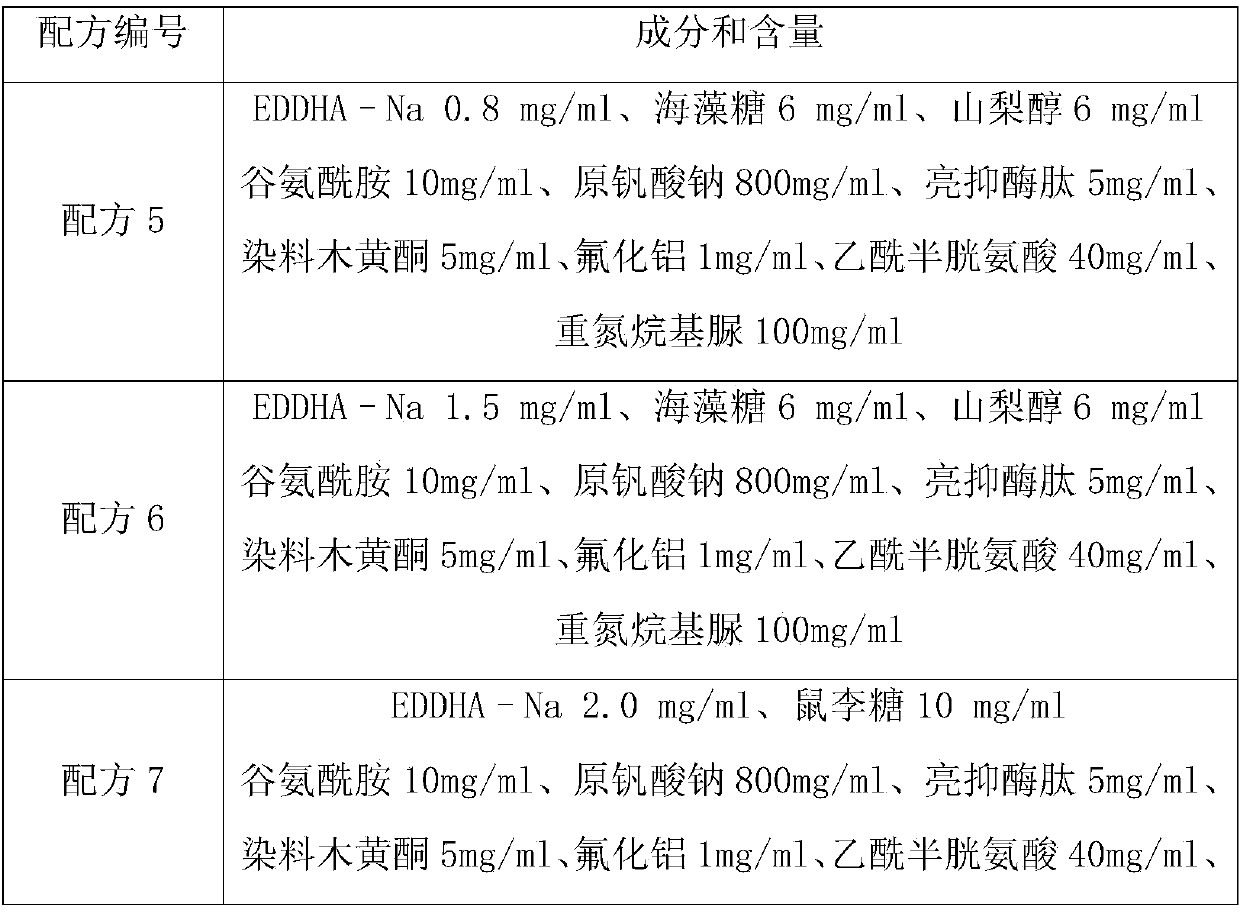
[0039] Table 2 Formulas based on EDDHA–Na anticoagulant
[0040]
[0041]
[0042] Table 3 Formulas based on EDTA-3K and EDDHA–Na anticoagulants
[0043]
[0044]
[0045] Fill the above nuclei...
Embodiment 2
[0046] Each formula performance comparison of embodiment 2
[0047]Recruit 3 male volunteers aged 22-25 (after physical examination: normal blood routine indicators, no physical examination tumor indications, no inflammation indications), blood collection 170ml (formula 1-14 blood collection tubes, Kangjieheparin lithium blood collection tubes, Streck non-invasive vacuum blood collection tube, blank glass blood collection tube). Store in a thermostat at room temperature at 23°C until the experiment begins.
[0048] Perform the following tests on each sample on days 0 (within 2 hours after blood collection), 2, 5, 10, 14, and 21:
[0049] For hemolysis, check the 414nm OD value. Calculate the three sample average.
[0050] According to the instructions of the kit, use the QIAamp Circulating Nucleic Acid Kit cfDNA Extraction Kit to extract the cfDNA in the sample, and use the qPCR method to detect the total concentration of cfDNA in the sample. The detection object is GAPDH, ...
Embodiment 3
[0060] Embodiment 3 preferred formula actually detects application
[0061] The vacuum blood collection tubes made of formula 3 and formula 12 preservative of the present application are used together with Streck non-invasive vacuum blood collection tubes for the actual detection of EGFR T790M in tumor patients (QIAamp Circulating Nucleic Acid Kit kit extracts cfDNA, EGFR Mutation Test kit detection, operation according to the kit standard procedure), involving a total of 40 patients, each patient used three kinds of blood collection tubes for sampling, the first test was performed within 48 hours after blood collection, stored at room temperature for 14 days and 21 days and then two test and compare the test results.
[0062] Table 6 EGFR T790M detection results
[0063]
[0064]
[0065] Except that the test results of the three storage methods within 48 hours and on the 14th in the formula 3 group were consistent, there were 3 cases of inconsistency (false positive)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com