Keratinase mutant with improved catalytic performance and application
A kind of keratinase mutation, keratinase technology, applied in the directions of application, hydrolase, biochemical equipment and method, can solve the problems of high production cost, low enzyme production level and the like, and achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the method for expressing KerBp in Bacillus subtilis system
[0027] Use gene-specific primers designed according to the coding sequence (SEQ ID NO.1) to amplify KerBp from the genome of Bacillus pumilus, connect the gel-purified PCR product to the pMD19-T simple vector, and then transform the ligated product into The clone host was Escherichia coli JM109, and cultured on LB agar plates containing ampicillin resistance (100 μg / mL) for 12 hours at 37°C. Positive colonies were inoculated into 10 mL of liquid LB containing ampicillin resistance (100 μg / mL) and cultured for 10-12 hours, plasmids were extracted and verified by DNA sequencing (Sangon Biotech). The cloning plasmid and expression vector pMA5 were digested with restriction endonucleases BamHI and Mlu I, then ligated with T4 DNA ligase at 16°C for 8 hours to construct the expression plasmid kerBp-pMA5, and transformed into the cloning host Escherichia coli JM109 by heat shock method In, the express...
Embodiment 2
[0028] Embodiment 2: fermentation culture condition and keratinase assay method
[0029] The recombinant strain was cultured at 37°C for 12h in 10 mL of LB medium containing kana resistance (100 μg / mL). Subsequently, the seed solution was inoculated into 50 mL of TB medium containing kana resistance (100 μg / mL), and further cultured at 37° C. for 48 h. Bacillus subtilis WB600 carrying pMA5 (used as a control group) was also cultured under the same conditions. The fermentation broth was centrifuged at 12,000 rpm for 5 min, and the supernatant was collected for activity determination and SDS-PAGE analysis.
[0030] Enzyme activity was determined at pH 9.0 and 40°C using 1% soluble keratin as substrate. The reaction mixture contained 1 mL of enzyme and 1 mL of 1% soluble keratin diluted in Tris-HCl buffer (0.05M, pH 9.0). The reaction was carried out in a water bath at 40° C. for 15 min, and the reaction was terminated with 2 mL of 5% TCA. The mixture was centrifuged at 12000r...
Embodiment 3
[0031] Example 3: Verification of the role of the leader peptide
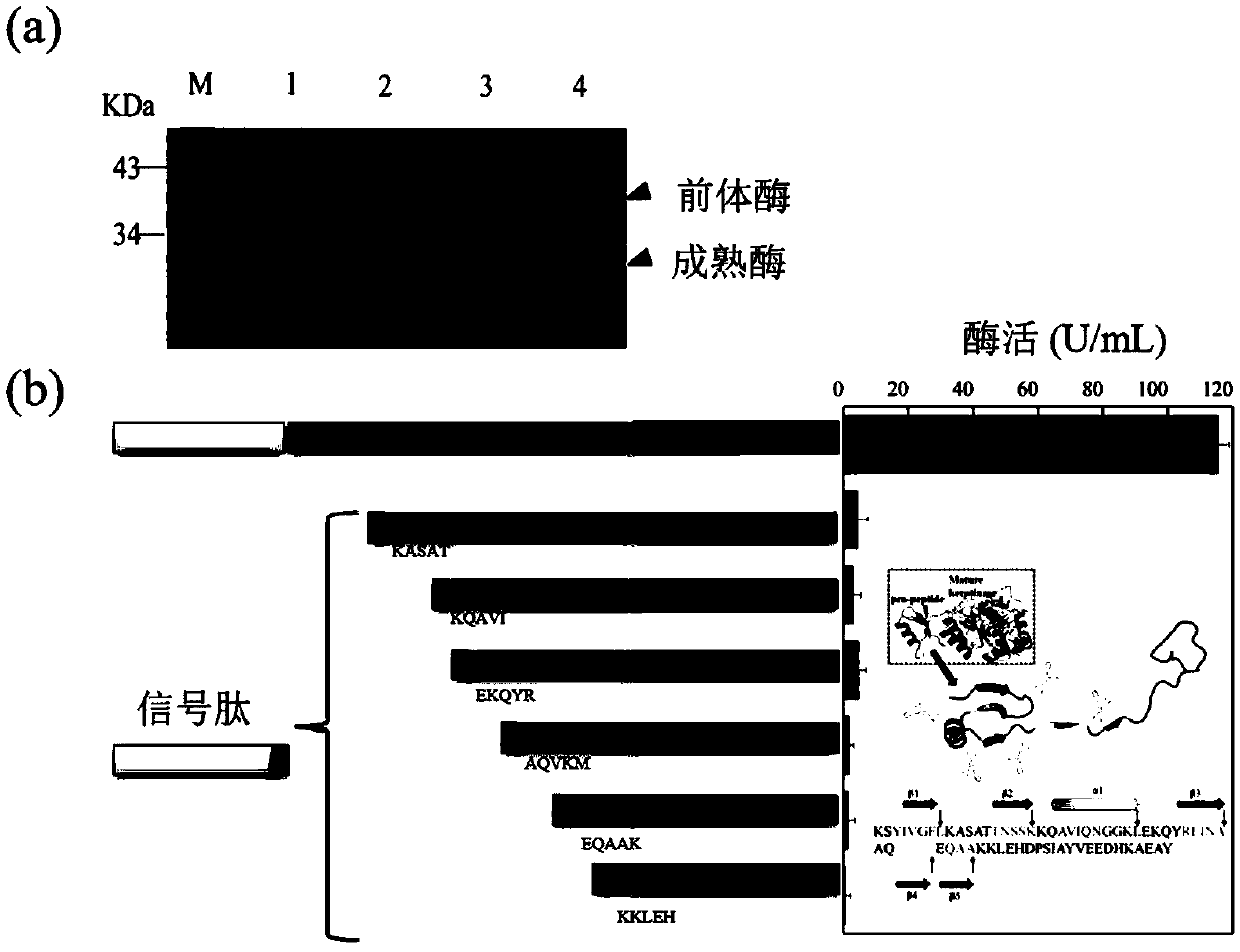
[0032] Using the plasmid kerBp-pMA5 as a template, the signal and mature keratinase were amplified by primers Signal(F) / Signal(D) and ker(F) / ker(D), respectively, to construct the leader peptide-deficient expression plasmid SMF-pMA5. Carry out cloning in Escherichia coli JM109, then extract plasmid, express in Bacillus subtilis WB600, through SDS-PAGE analysis, observe that molecular weight is the protein band of 30kDa, but do not detect keratinase activity ( figure 2 a).
[0033] Using the kerBp-pMA5 plasmid as a template, the signal peptide sequence and the truncated mutant sequence were respectively amplified by designing primers with different homology arms, and then the signal peptide was added to the N-terminus of the mature enzyme to obtain recombination with different leader peptide lengths Plasmids, expressed in Bacillus subtilis WB600, and assayed for activity. The results of the activity assay sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com